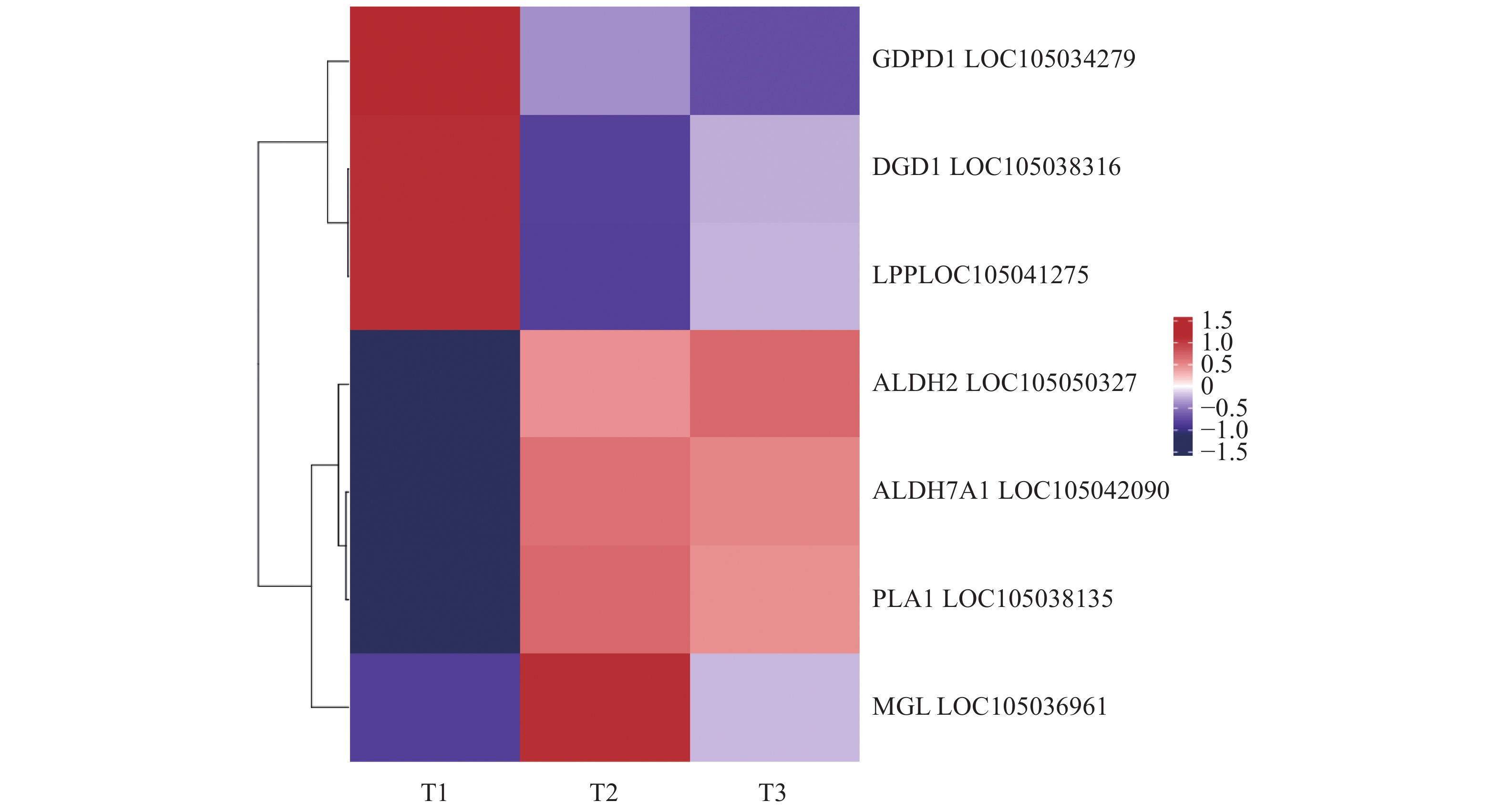
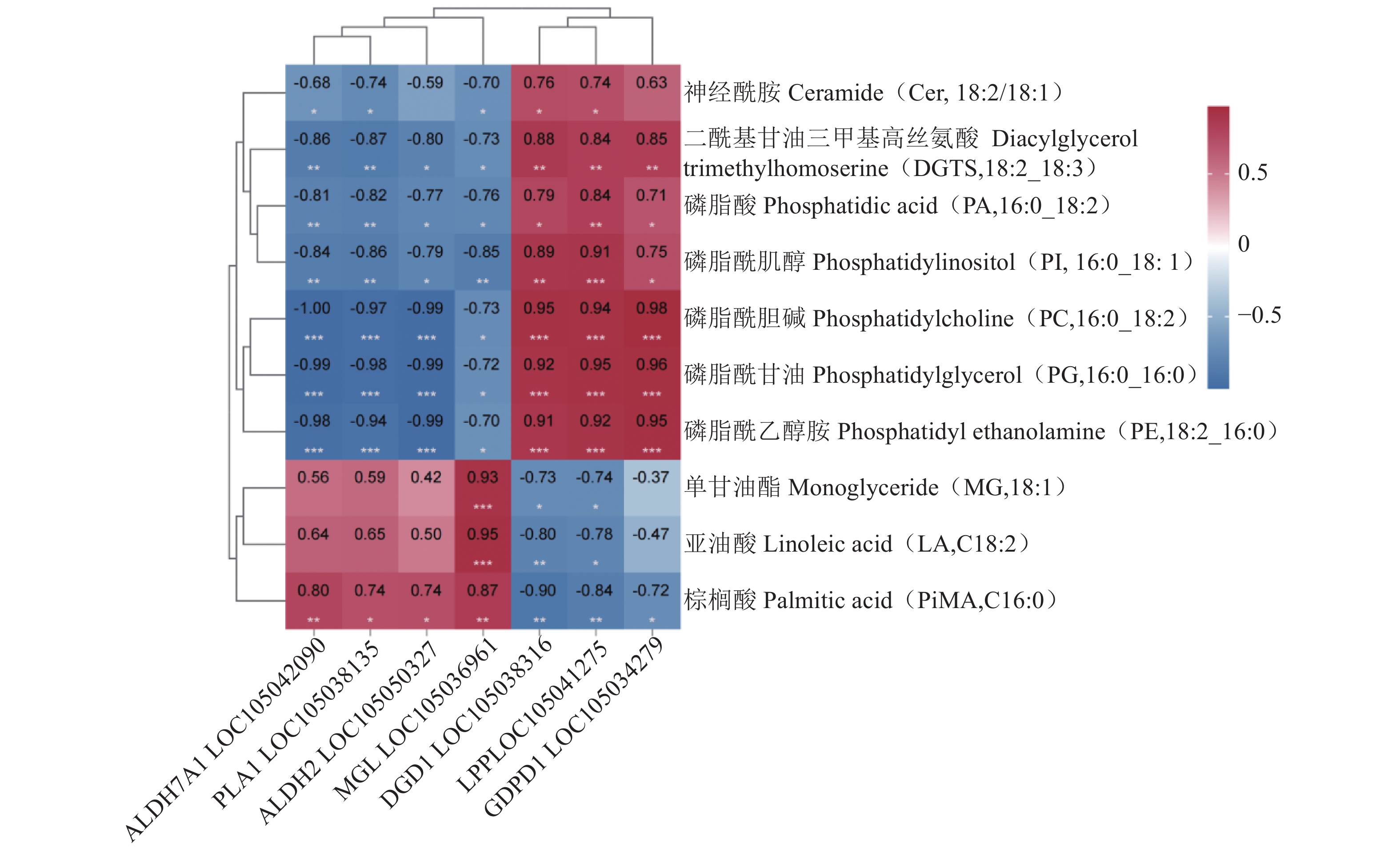
Differentiation in Post-harvest Lipid Metabolism of Oil Palm Fruits
-
摘要:
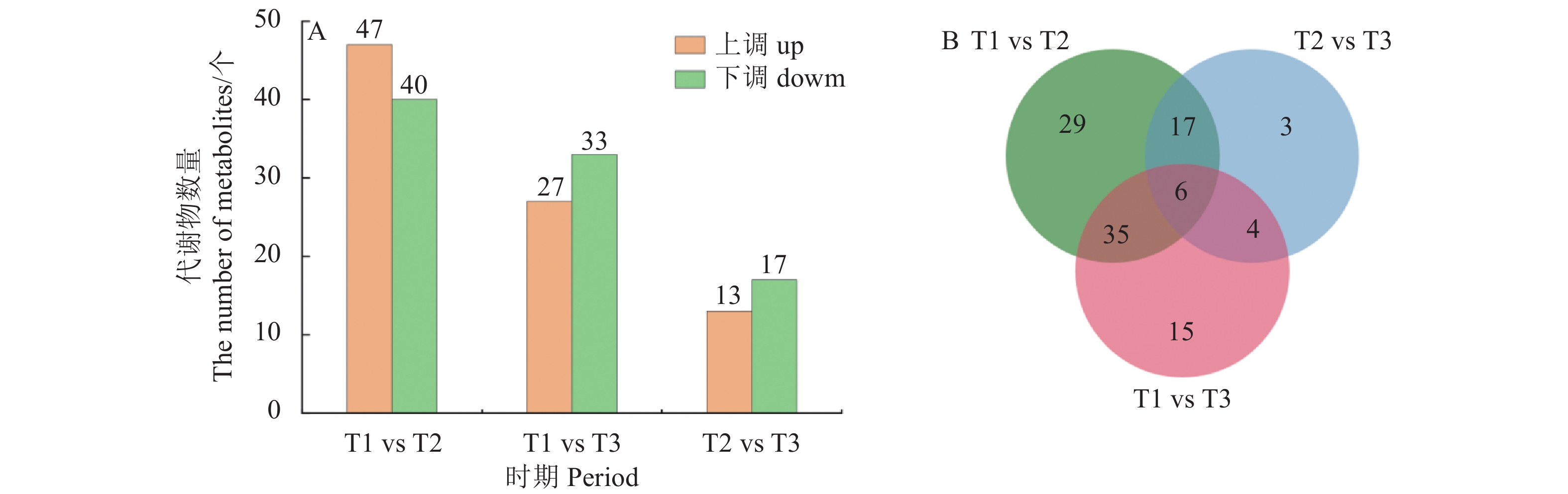
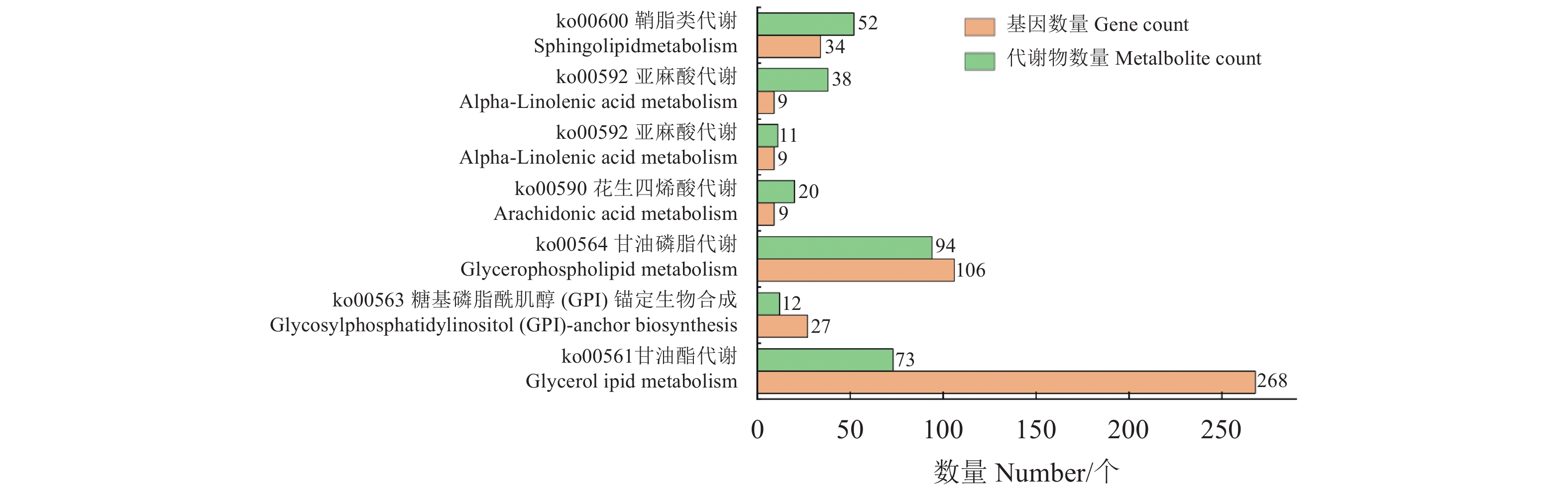
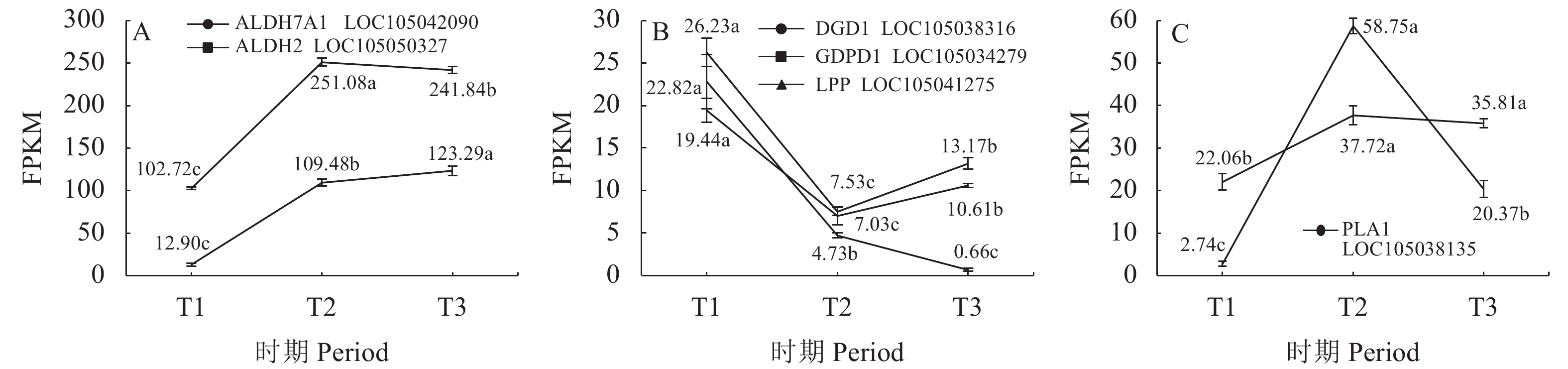
目的 探究薄壳种油棕果实采后阶段脂质合成和积累的机制。 方法 选用采后不同处理时间的薄壳型油棕果实[授粉后185 d刚采摘的鲜果(T1)、采收后24 h(T2)、采收后36 h(T3)],结合液相色谱-串联质谱法(Liquid chromatography-tandem mass spectrometry, LC-MS/MS)和RNA测序(RNA sequencing, RNA-seq)技术,对各脂质代谢物和差异表达基因在酸败过程中的动态变化进行测定和分析。 结果 在采后不同处理时间的果实脂质中共鉴定出5个脂质大类、23个脂质亚类,520个脂质单体分子。联合分析结果表明,乙醛脱氢酶(ALDH7A1、ALDH2)、单酰甘油脂肪酶(MGL)和磷脂酶A1(PLA1)与二酰基甘油三甲基高丝氨酸(DGTS)、磷脂酸(PA)、磷脂酰肌醇(PI)、磷脂酰胆碱(PC)、磷脂酰甘油(PG)、磷脂酰乙醇胺(PE)等甘油磷脂类物质分别均呈显著负相关,与棕榈酸等游离脂肪酸呈显著正相关;甘油磷酸二酯磷酸二酯酶(GDPD1)、脂磷酸磷酸酶(LPP)和二半乳糖甘油酯合成酶(DGD1)与DGTS、PA、PI、PC、PG、PE等甘油磷脂类物质呈显著正相关,与棕榈酸等游离脂肪酸呈显著负相关;MGL与单甘油酯(MG)和亚油酸(LA)呈极显著正相关,与神经酰胺(Cer)呈显著负相关;DGD1和LPP与MG和LA呈极显著负相关,与Cer呈显著正相关。 结论 ALDH7A1、ALDH2、PLA1、MGL可能抑制甘油磷脂类物质的合成,促进棕榈酸等脂肪酸物质的合成;DGD1、LPP和GDP1可能促进甘油磷脂类物质的合成,抑制棕榈酸等脂肪酸物质的合成。 Abstract:Objective Mechanism and accumulation of lipid synthesis in thin-shelled oil palm fruits was investigated for breeding of variety resistant to fat rancidity. Method Fruits of thin-shelled oil palm freshly harvested 185d after pollination (T1), 24h post-harvest (T2), and 36h post-harvest (T3) were collected for LC-MS/MS and RNA-seq determination and analysis on lipid metabolites and differentially expressed genes in mesocarp of oil palm fruits as the lipid oxidation taking place. [Result] In the fruit development, 5 lipid classes, 23 lipid subclasses, and 520 monomer molecules in mesocarp of the oil palm fruits were identified. It is well known that the hydrolysis of phosphatidylcholine (PC) is a lipid oxidation and the hydrolyzed glycerophosphate choline (GPC) affects PC, lipophosphatase (LPP) promotes synthesis of phosphates and glycerophospholipids, and the expression of chlorophyll relates to chlorophyll content. This study found that the aldehyde dehydrogenase (ALDH7A1 and ALDH2), monoacylglycerol lipase (MGL), phospholipase A1 (PLA1), and glycerophosphodiester phosphodiesterase (GDPD1) significantly negatively correlated with glycerophospholipids, such as diacylglycerol trimethyl homoserine (DGTS), phosphatidic acid (PA), phosphatidylinositol (PI), phosphatidylcholine (PC), phosphatidylglycerol (PG), phosphatidylethanolamine (PE), but significantly positively correlated with palmitic acid, while GDPD1, LPP, and digalactosylglycerol synthase (DGD1) significantly positively correlated with the glycerophospholipids, such as DGTS, PA, PI, PC, PG, PE, but negatively correlated with palmitic acid, whereas MGL monoglyceride (MG) extremely significantly positively correlated with linoleic acid (LA) but significantly negatively correlated with ceramide (Cer), and DGD1 and LPP significantly negatively correlated with MG and LA, but significantly positively correlated with Cer. Conclusion It appeared that ALDH7A1, ALDH2, PLA1, and MGL inhibited the glycerophospholipids synthesis but promoted the synthesis of fatty acids such as palmitic acid, while DGD1, LPP, and GDP1 enhanced the synthesis of glycerophospholipids but retarded that of palmitic and other fatty acids. -
Key words:
- Oil palm fruit /
- rancidity /
- lipid /
- metabolomics /
- transcriptomics
-
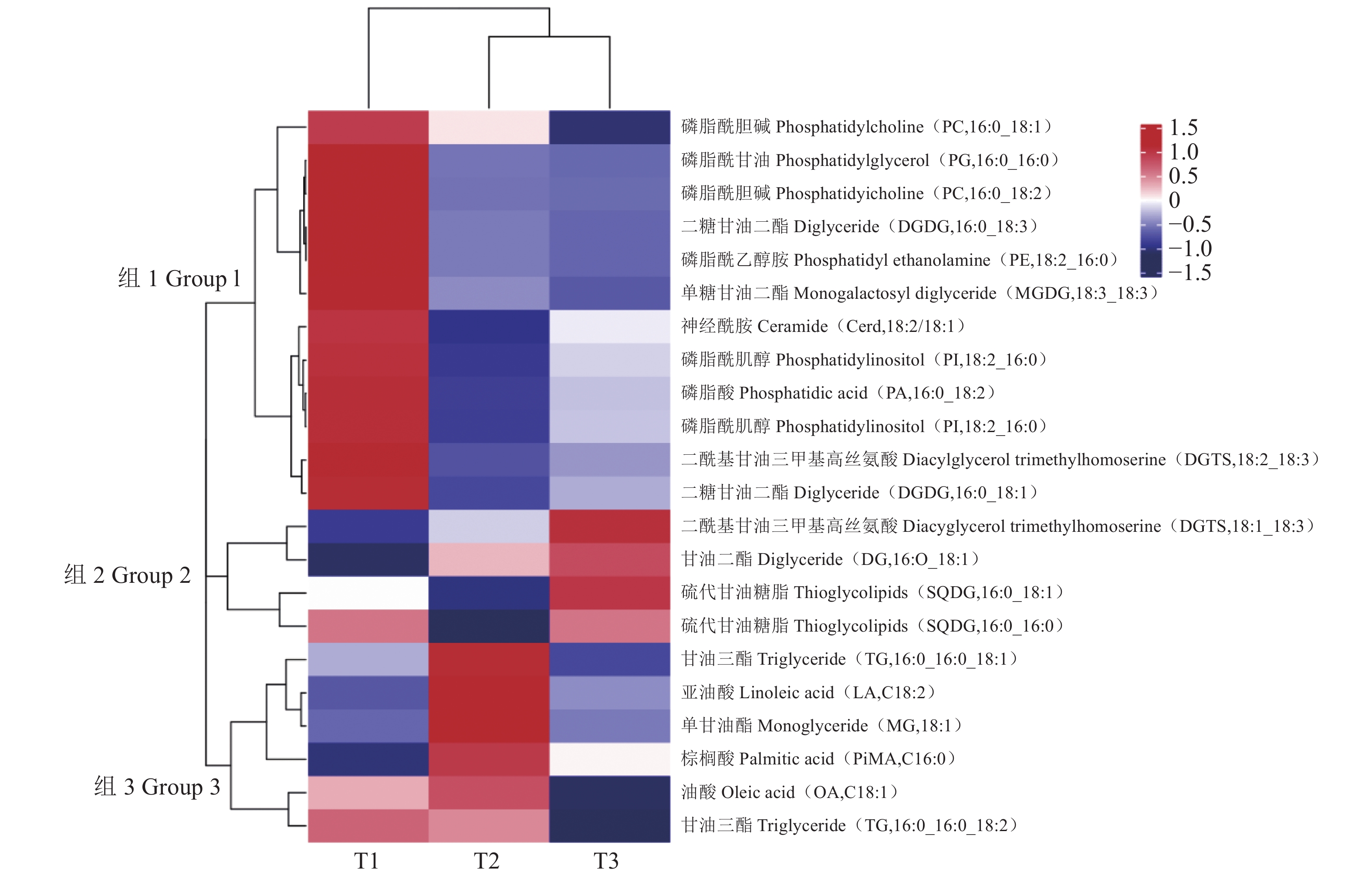
表 1 采后不同处理时间油棕中果皮中各亚类脂质含量
Table 1. Lipid content of subclasses in rancid oil palm fruit mesocarp
(单位:nmol·g−1) 大类
Large category亚类
Subclass采后不同处理时间
Different post harvest processing timesT1 T2 T3 鞘脂类(SP) 神经酰胺(Cer) 28.94±3.85a 21.53±0.51b 25.84±4.13ab 鞘氨醇(SPH) 3.60±0.59b 4.18±0.44ab 5.29±0.59a 甘油酯(GL) 甘油二脂(DG) 315345.99 ±61101.54 460114.66 ±90158.07 450646.11 ±191075.41 单甘油酯(MG) 1946.23 ±288.28b4053.17 ±336.16a2043.03 ±291.06b甘油三酯(TG) 24509.04 ±2703.78 29153.02 ±3638.80 25915.44 ±6506.09 糖脂(SL) 二糖甘油二酯(DGDG) 2743.20 ±435.702248.80 ±555.862299.76 ±1216.49 糖鞘脂(HexCer) 5.00±0.05a 5.29±0.39a 4.19±0.22b 单糖甘油二酯(MGDG) 1058.30 ±200.41745.27±95.96 809.81±378.32 硫代甘油糖脂(SQDG) 842.04±149.80 713.06±124.43 933.09±491.32 甘油磷脂(GP) 二酰基甘油三甲基高丝氨酸(DGTS) 444.84±94.94 328.17±55.95 428.34±229.02 溶血二酰基甘油基三甲基高丝氨酸(LDGTS) 14.77±4.15 15.79±4.04 29.49±14.31 溶血磷脂酸(LPA) 25.35±0.90c 40.73±0.11b 53.25±6.90a 溶血磷脂酰胆碱(LPC) 91.30±2.95c 121.40±4.41b 139.27±10.88a 溶血磷脂酰乙醇胺(LPE) 46.51±1.85c 64.48±1.47b 88.26±9.44a 溶血磷脂酰甘油(LPG) 7.26±0.16b 12.00±0.57a 12.43±1.72a 溶血磷脂酰肌醇(LPI) 2.52±0.05b 9.31±1.31b 49.10±15.01a 磷脂酸(PA) 3706.34 ±225.693320.89 ±586.403715.32 ±506.73磷脂酰胆碱(PC) 1339.82 ±63.09a816.66±32.87b 815.64±42.47b 磷脂酰乙醇胺(PE) 1155.08 ±80.28s604.46±34.88b 582.75±72.54b 磷脂酰甘油(PG) 991.61±56.31a 524.11±4.14b 485.01±4.77b 磷脂酰肌醇(PI) 654.01±32.33ab 453.29±34.92b 540.57±70.70b 磷脂酰甲醇(PMeOH) 5.50±0.17a 2.05±0.08b 1.28±0.10c 脂肪酰类(FA) 游离脂肪酸(FFA) 60174.75 ±4469.14b73407.38 ±2827.63a60715.71 ±476.04b数值为平均数±标准差,不同小写字母表示不同时期之间差异显著(P<0.05)。表2同。
Values are mean ± standard deviation; different lowercase letters indicates significant differences of different treatment times (P<0.05).表 2 采后不同处理时间油棕中果皮中22种脂质单体分子含量
Table 2. Molecular contents of 22 lipid monomers in rancid oil palm fruit mesocarp
(单位:nmol·g−1) 物质 Substance 采后不同处理时间 Different post harvest processing times T1 T2 T3 神经酰胺 (Cer, 18:2/18:1) 14.52±2.31a 10.66±0.58b 12.41±1.91ab 甘油二酯(DG, 16:0_18:1) 70636.96 ±15009.08 92432.42 ±18944.80 101472.36 ±51725.27 单甘油酯(MG, 18:1) 1403.68 ±224.40b2965.58 ±262.87a1485.12 ±220.97b甘油三酯(TG, 16:0_16:0_18:1) 3516.58 ±764.074308.94 ±930.543231.80 ±1569.97 甘油三酯(TG, 16:0_16:0_18:2) 2629.11 ±346.552608.90 ±543.372440.06 ±446.34二糖甘油二酯(DGDG, 16:0_18:1) 778.55±99.30 612.69±142.67 656.75±368.55 二糖甘油二酯(DGDG, 16:0_18:3) 485.56±86.07 342.74±76.95 333.41±135.86 单糖甘油二酯(MGDG, 18:3_18:3) 324.09±76.51 251.13±55.70 239.09±98.34 硫代甘油糖脂(SQDG, 16:0_18:1) 299.17±43.36 244.37±43.76 356.16±191.01 硫代甘油糖脂(SQDG, 16:0_16:0) 422.55±89.76 335.69±56.08 422.63±217.14 二酰基甘油三甲基高丝氨酸(DGTS, 18:1_18:3) 104.66±17.65 115.37±24.87 133.59±76.09 二酰基甘油三甲基高丝氨酸(DGTS, 18:2_18:3) 124.13±26.63a 48.65±4.42b 62.68±31.21b 磷脂酸(PA, 16:0_18:2) 1354.25 ±101.53a919.47±176.05b 1063.04 ±145.54ab磷脂酰胆碱(PC, 16:0_18:1) 412.13±29.49 390.23±17.30 361.94±27.71 磷脂酰胆碱(PC, 16:0_18:2) 458.78±21.04a 144.72±9.91b 137.97±10.89b 磷脂酰乙醇胺(PE, 18:2_16:0) 505.79±32.61a 225.63±15.30b 207.44±29.24b 磷脂酰甘油(PG, 16:0_16:0) 524.14±36.45a 191.76±5.24b 179.77±9.85b 磷脂酰肌醇(PI, 16:0_18:1) 344.68±14.83a 230.59±17.54b 274.07±44.09b 磷脂酰肌醇(PI, 18:2_16:0) 164.18±10.24a 119.57±10.73b 134.77±13.99b 亚油酸(LA, C18:2) 10188.00 ±782.31b15804.62 ±524.96a11046.89 ±836.18b棕榈酸(PiMA, C16:0) 14345.15 ±1055.52b17834.69 ±819.45a16177.21 ±580.58a油酸(OA, C18:1) 30396.86 ±2655.33a31704.01 ±568.28a26683.76 ±797.90b -
[1] MURPHY D J. Oil palm: Future prospects for yield and quality improvements [J]. Lipid Technology, 2009, 21(11/12): 257−260. [2] CORLEY R H V, TINKER P B. The Oil Palm[M]. Oxford, United Kingdom: Blackwell Pub Professional, 2003.CORLEY R H V, TINKER P B. The Oil Palm[M]. Oxford, United Kingdom: Blackwell Pub Professional, 2003. [3] PARVEEZ G K, RASID O A, MASANI M Y A, et al. Biotechnology of oil palm: Strategies towards manipulation of lipid content and composition [J]. Plant Cell Reports, 2015, 34(4): 533−543. doi: 10.1007/s00299-014-1722-4 [4] HOU Q C, UFER G, BARTELS D. Lipid signalling in plant responses to abiotic stress[J]. Plant, Cell & Environment, 2016, 39(5): 1029-1048. [5] GROUP L T. Comprehensive classification system for lipids published[J]. Lipid Technology: the International Magazine of Oils, Fats, Lipids & Waxes, 2005, 17(8): 187. [6] PATI S, NIE B, ARNOLD R D, et al. Extraction, chromatographic and mass spectrometric methods for lipid analysis [J]. Biomedical Chromatography, 2016, 30(5): 695−709. doi: 10.1002/bmc.3683 [7] CHEONG W F, WENK M R, SHUI G H. Comprehensive analysis of lipid composition in crude palm oil using multiple lipidomic approaches [J]. Journal of Genetics and Genomics, 2014, 41(5): 293−304. doi: 10.1016/j.jgg.2014.04.002 [8] Fabienne Bourgis, Aruna Kilaru, Xia Cao, Georges-Frank Ngando-Ebongue, Noureddine Drira, John B. Ohlrogge and Vincent Arondel (2011). Comparative transcriptome and metabolite analysis of oil palm and date palm mesocarp that differ dramatically in carbon partitioning. Proceedings of the National Academy of Sciences of the United States of America, 108(30), 12527–12532. doi: 10.2307/27979035 [9] TRANBARGER T J, DUSSERT S, JOËT T, et al. Regulatory mechanisms underlying oil palm fruit mesocarp maturation, ripening, and functional specialization in lipid and carotenoid metabolism [J]. Plant Physiology, 2011, 156(2): 564−584. doi: 10.1104/pp.111.175141 [10] LU C F, XIN Z G, REN Z H, et al. An enzyme regulating triacylglycerol composition is encoded by the ROD1 gene of Arabidopsis [J]. Proceedings of the National Academy of Sciences of the United States of America, 2009, 106(44): 18837−18842. [11] Bourgis F , Kilaru A , Cao X ,et al.Comparative transcriptome and metabolite analysis of oil palm and date palm mesocarp that differ dramatically in carbon partitioning[J].Proceedings of the National Academy of Sciences of the United States of America, 2011, 108(30):p.12527-12532.DOI: 10.1073/pnas.1106502108 [12] NAKAMURA Y, TSUCHIYA M, OHTA H. Plastidic phosphatidic acid phosphatases identified in a distinct subfamily of lipid phosphate phosphatases with prokaryotic origin [J]. Journal of Biological Chemistry, 2007, 282(39): 29013−29021. doi: 10.1074/jbc.M704385200 [13] 雷新涛, 曹红星. 油棕[M]. 北京: 中国农业出版社, 2013. [14] Naim S , Missihoun T D , Kotchoni S O , et al. Aldehyde Dehydrogenases in Arabidopsis thaliana: Biochemical Requirements, Metabolic Pathways, and Functional Analysis[J]. Frontiers in Plant Science, 2011, (2): 65. DOI: 10.3389/fpls.2011.00065. [15] CHEN Z, CHEN M, XU Z S, et al. Characteristics and expression patterns of the aldehyde dehydrogenase (ALDH) gene superfamily of foxtail millet (Setaria italica L. ) [J]. PLoS One, 2014, 9(7): e101136. doi: 10.1371/journal.pone.0101136 [16] VASILIOU V, NEBERT D W. Analysis and update of the human aldehyde dehydrogenase (ALDH) gene family [J]. Human Genomics, 2005, 2(2): 138−143. doi: 10.1186/1479-7364-2-2-138 [17] BROCKER C, VASILIOU M, CARPENTER S, et al. Aldehyde dehydrogenase (ALDH) superfamily in plants: Gene nomenclature and comparative genomics [J]. Planta, 2013, 237(1): 189−210. doi: 10.1007/s00425-012-1749-0 [18] ABDUL W, ALIYU S R, LIN L L, et al. Family-four aldehyde dehydrogenases play an indispensable role in the pathogenesis of Magnaporthe oryzae [J]. Frontiers in Plant Science, 2018, 9: 980. doi: 10.3389/fpls.2018.00980 [19] LI Z, WANG J Y, LONG H X, et al. Cloning and expression analysis of an aldehyde dehydrogenase gene from Camellia oleifera [J]. Nanoscience and Nanotechnology Letters, 2017, 9(3): 364−373. doi: 10.1166/nnl.2017.2340 [20] TAGNON M D, SIMEON K O. Aldehyde dehydrogenases may modulate signaling by lipid peroxidation-derived bioactive aldehydes [J]. Plant Signaling & Behavior, 2017, 12(11): e1387707. [21] BARTELS D, SUNKAR R. Drought and salt tolerance in plants [J]. Critical Reviews in Plant Sciences, 2005, 24(1): 23−58. doi: 10.1080/07352680590910410 [22] 杨程, 张淑岩, 韦露, 等. 薄壳种油棕果实发育和采后脂肪酸合成转录代谢差异分析[J/OL]. 分子植物育种, 2023 (2023-06-13). https://kns.cnki.net/kcms/detail/46.1068.S.20230612.1612.020.html.YANG C, ZHANG S Y, WEI L, et al. Differential Analysis of Fatty Acid Synthesis, Transcriptional Metabolism during Fruit Development and Postharvest in Tenera Oil palm[J/OL]. Molecular Plant Breeding, 2023 (2023-06-13). https://kns.cnki.net/kcms/detail/46.1068.S.20230612.1612.020.html.(in Chinese) [23] RIEGLER-BERKET L, LEITMEIER A, ASCHAUER P, et al. Identification of lipases with activity towards monoacylglycerol by criterion of conserved cap architectures [J]. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids, 2018, 1863(7): 679−687. doi: 10.1016/j.bbalip.2018.03.009 [24] KIM R J, KIM H J, SHIM D, et al. Molecular and biochemical characterizations of the monoacylglycerol lipase gene family of Arabidopsis thaliana [J]. The Plant Journal, 2016, 85(6): 758−771. doi: 10.1111/tpj.13146 [25] MARIANI M E, FIDELIO G D. Secretory phospholipases A2 in plants [J]. Frontiers in Plant Science, 2019, 10: 861. doi: 10.3389/fpls.2019.00861 [26] RYU S B. Phospholipid-derived signaling mediated by phospholipase A in plants [J]. Trends in Plant Science, 2004, 9(5): 229−235. doi: 10.1016/j.tplants.2004.03.004 [27] WANG X M. Plant phospholipases [J]. Annual Review of Plant Physiology and Plant Molecular Biology, 2001, 52: 211−231. doi: 10.1146/annurev.arplant.52.1.211 [28] 史敬芳, 张琪, 宋松泉, 等. 磷脂酶及其调控种子活力研究进展 [J]. 南方农业学报, 2022, 53(9):2612−2623. doi: 10.3969/j.issn.2095-1191.2022.09.024SHI J F, ZHANG Q, SONG S Q, et al. Phospholipases and their seed vigor regulation: A review [J]. Journal of Southern Agriculture, 2022, 53(9): 2612−2623. (in Chinese) doi: 10.3969/j.issn.2095-1191.2022.09.024 [29] MUKHERJEE A B. Biochemistry, molecular biology, and physiology of phospholipase A2 and its regulatory factors [J]. Advances in Experimental Medicine and Biology, 1990, 279: 1−251. [30] LIM C W, KIM B H, KIM I H, et al. Modeling and optimization of phospholipase A1-catalyzed hydrolysis of phosphatidylcholine using response surface methodology for lysophosphatidylcholine production [J]. Biotechnology Progress, 2015, 31(1): 35−41. doi: 10.1002/btpr.2009 [31] ZHAO Q Y, WANG M M, ZHANG W B, et al. Impact of phosphatidylcholine and phosphatidylethanolamine on the oxidative stability of stripped peanut oil and bulk peanut oil [J]. Food Chemistry, 2020, 311: 125962. doi: 10.1016/j.foodchem.2019.125962 [32] CHENG Y X, ZHOU W B, EL SHEERY N I, et al. Characterization of the Arabidopsis glycerophosphodiester phosphodiesterase (GDPD) family reveals a role of the plastid-localized AtGDPD1 in maintaining cellular phosphate homeostasis under phosphate starvation [J]. The Plant Journal, 2011, 66(5): 781−795. doi: 10.1111/j.1365-313X.2011.04538.x [33] BANG H J, KIM I H, KIM B H. Phospholipase A1-catalyzed hydrolysis of soy phosphatidylcholine to prepare l-α-glycerylphosphorylcholine in organic-aqueous media [J]. Food Chemistry, 2016, 190: 201−206. doi: 10.1016/j.foodchem.2015.05.093 [34] CARMAN G M. Phosphatidate phosphatases and diacylglycerol pyrophosphate phosphatases in Saccharomyces cerevisiae and Escherichia coli [J]. Biochimica et Biophysica Acta, 1997, 1348(1/2): 45−55. [35] MUNNIK T, LIGTERINK W, et al. Distinct osmo-sensing protein kinase pathways are involved in signalling moderate and severe hyper-osmotic stress [J]. The Plant Journal, 1999, 20(4): 381−388. doi: 10.1046/j.1365-313x.1999.00610.x [36] LIU Z F, YAN H C, WANG K B, et al. Crystal structure of spinach major light-harvesting complex at 2.72 A resolution [J]. Nature, 2004, 428(6980): 287−292. doi: 10.1038/nature02373 [37] JORDAN P, FROMME P, WITT H T, et al. Three-dimensional structure of cyanobacterial photosystem I at 2.5 Å resolution [J]. Nature, 2001, 411: 909−917. doi: 10.1038/35082000 [38] UMENA Y, KAWAKAMI K, SHEN J R, et al. Crystal structure of oxygen-evolving photosystem II at a resolution of 1.9 Å [J]. Nature, 2011, 473: 55−60. doi: 10.1038/nature09913 [39] KELLY A A, FROEHLICH J E, DÖRMANN P. Disruption of the two digalactosyldiacylglycerol synthase genes DGD1 and DGD2 in Arabidopsis reveals the existence of an additional enzyme of galactolipid synthesis [J]. The Plant Cell, 2003, 15(11): 2694−2706. doi: 10.1105/tpc.016675 [40] KOBAYASHI K, FUJII S, SASAKI D, et al. Transcriptional regulation of thylakoid galactolipid biosynthesis coordinated with chlorophyll biosynthesis during the development of chloroplasts in Arabidopsis [J]. Frontiers in Plant Science, 2014, 5: 272. -







 下载:
下载: