A Constant Temperature Fluorescent RPA Assay for Detecting Novel Muscovy Duck Parvovirus
-
摘要:
目的 建立新型番鸭细小病毒(New-genotype muscovy duck parvovirus, N-MDPV)荧光RPA恒温检测方法,为基层提供可视化快速检测技术手段。 方法 以N-MDPV的VP3基因保守片段为靶点,使用EXO荧光探针特定结合VP3基因保守片段,设计特异的RPA扩增引物并利用重组酶聚合酶扩增技术扩增目的基因,从而建立一种荧光RPA恒温检测N-MDPV的方法,确定反应体系的最佳反应时间和温度,分析该方法特异性和灵敏性;对收集的病料进行核酸检测,并与传统PCR和病毒分离鉴定方法检测结果进行比较。 结果 建立的荧光RPA恒温检测方法最佳反应温度为39 ℃,最佳反应时间为30 min;灵敏性高,最低核酸检测限度可达10 fg·μL−1;对新型番鸭细小病毒核酸进行特异性扩增,结果显示对鸭腺病毒3型(Duck adenovirus 3, DAdV-3)、禽腺病毒4型(Fowl adenovirus serotype 4, FAdV-4)、鸭圆环病毒(Duck circovirus, DuCV)、鸭瘟病毒(Duck plaguevirus, DPV)、鸭病毒性肝炎病毒(Duck hepatitis Virus, DHV)、鸭坦布苏病毒(Duck tembusu virus, DTMUV)和新型鸭呼肠孤病毒(Novel duck reovirus, NDRV)的核酸均未有发生交叉反应,特异性良好。利用本研究建立的RPA快检方法、传统PCR方法以及病毒分离鉴定方法对收集的38份鸭组织病料核酸样品进行检测,结果显示阳性率分别为36.8%(14/38)、36.8%(14/38)和31.6%(12/38);RPA检测后呈阳性的样品经PCR方法检测与病毒分离鉴定方法检测均呈现阳性,阳性符合率为100%。临床样品检测RPA检测方法与传统PCR方法阳性符合率为100%。 结论 该方法可以很好地应用于缺乏相应检测设备的基层进行新型番鸭细小病毒的大规模临床样本检测,为新型番鸭细小病毒的可视化快速检测提供技术手段。 -
关键词:
- 新型番鸭细小病毒 /
- RPA恒温检测 /
- 重组酶聚合酶扩增技术
Abstract:Objective A constant temperature fluorescent RPA assay for detecting novel Muscovy duck parvovirus (N-MDPV) was developed. Method EXO fluorescent probes were specifically bound to the targeted conserved VP3 fragment of N-MDPV. RPA amplification primers were designed to amplify the segment by using recombinant enzyme polymerase. An assay for detecting N-MDPV was established with reaction time and temperature optimized and specificity, sensitivity, and accuracy tested on the collected nucleic acid of disease material in comparison with traditional PCR and virus isolation identification methods. Result The optimized assay reaction temperature and time were 39 ℃ for 30m to achieve a lowest sensitivity for nucleic acid detection at 10 fg·μl−1. The nucleic acid of N-MDPV was specifically amplified without any cross reaction with those of duck adenovirus type 3, avian adenovirus type 4, duck circovirus, duck plague virus, duck viral hepatitis virus, duck Tambousu virus, and novel duck reovirus. Along with the conventional PCR and the virus isolation and identification methods, the newly developed assay detected the nucleic acid on 38 duck tissue specimens with a positive rate of 36.8% (14/38) in comparison to those at 36.8% (14/38) for the PCR and 31.6% (12/38) for the isolation and identification method. In addition, 100% coincidence rates of the assay and the two other methods on positive samples as well as of the assay and the PCR on the positive clinical samples were secured. Conclusion The new RPA method to rapidly and visually detect N-MDPV demonstrated to be highly specific, sensitive, and accurate. It was deemed appropriate for clinical applications. -
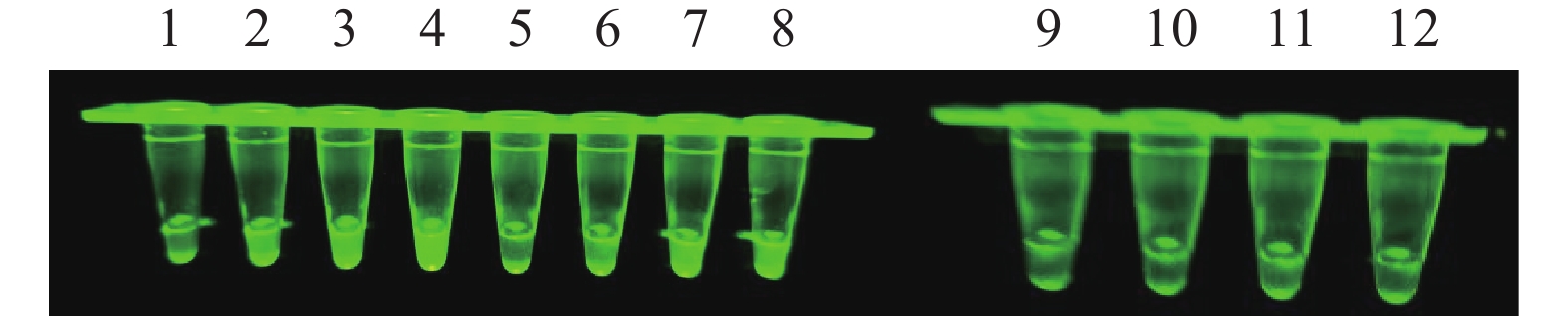
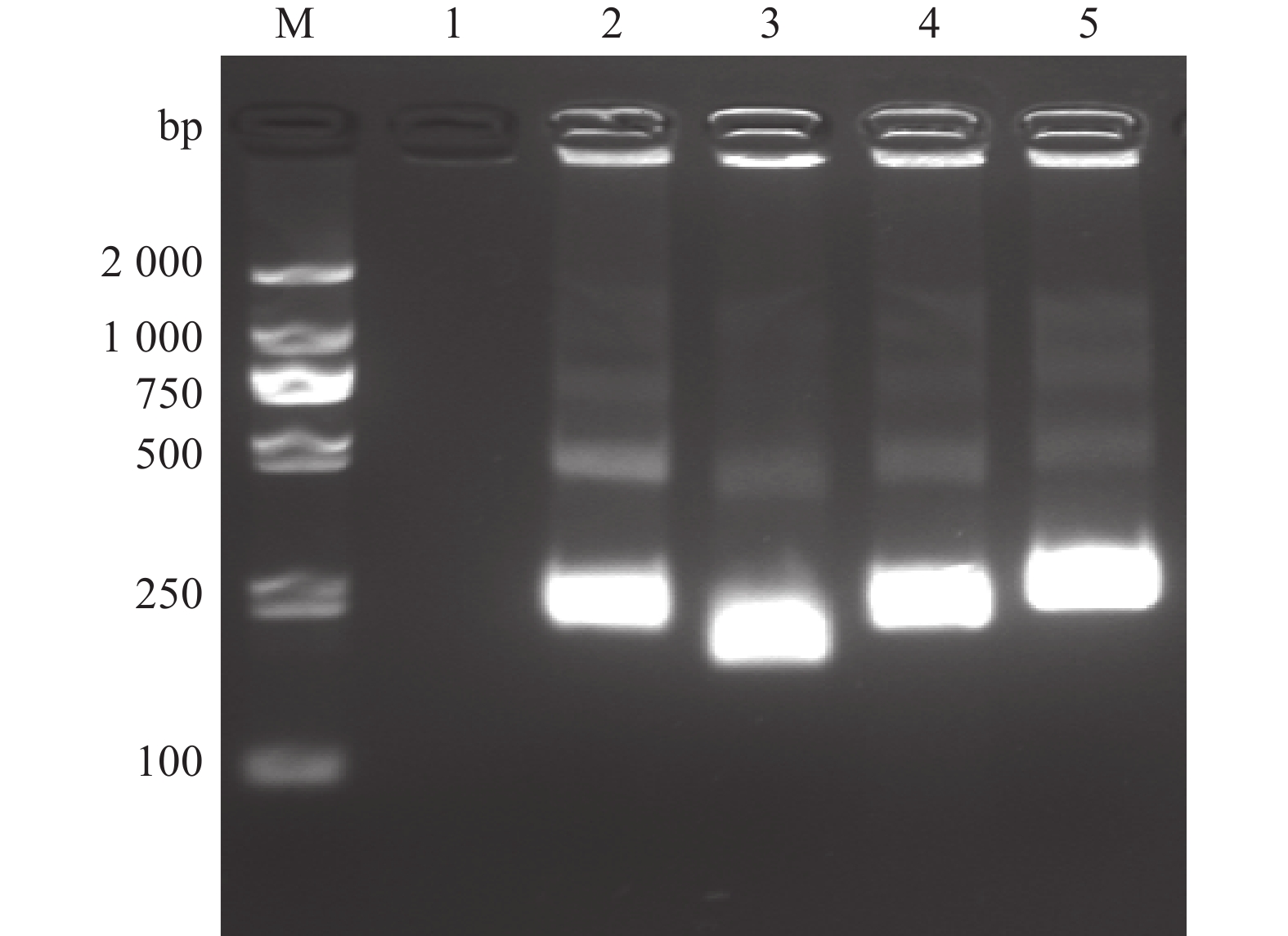
图 2 N-MDPV荧光RPA检测不同引物扩增结果
1~4(探针浓度4 μmol·L−1):N-MDPV-228F-228R、N-MDPV-193F-193R、N-MDPV-193F-230R、N-MDPV-193F-250R;5~8(探针浓度2 μmol·L−1):N-MDPV-228F-228R、N-MDPV-193F-193R、N-MDPV-193F-230R、N-MDPV-193F-250R;9~12(阴性对照):N-MDPV-228F-228R、N-MDPV-193F-193R、N-MDPV-193F-230R、N-MDPV-193F-250R。
Figure 2. Amplification of primers of RPA for N-MDPV
1–4 (with probe concentration at 4μmol·L−1): N-MDPV-228F-228R, N-MDPV-193F-193R, N-MDPV-193F-230R, N-MDPV-193F-250R, respectively; 5–8 (with probe concentration at 2μmol·L−1): N-MDPV-228F-228R, N-MDPV-193F-193R, N-MDPV-193F-230R, N-MDPV-193F-250R, respectively; 9–12 (negative control): N-MDPV-228F-228R, N-MDPV-193F-193R, N-MDPV-193F-230R, N-MDPV-193F-250R, respectively.
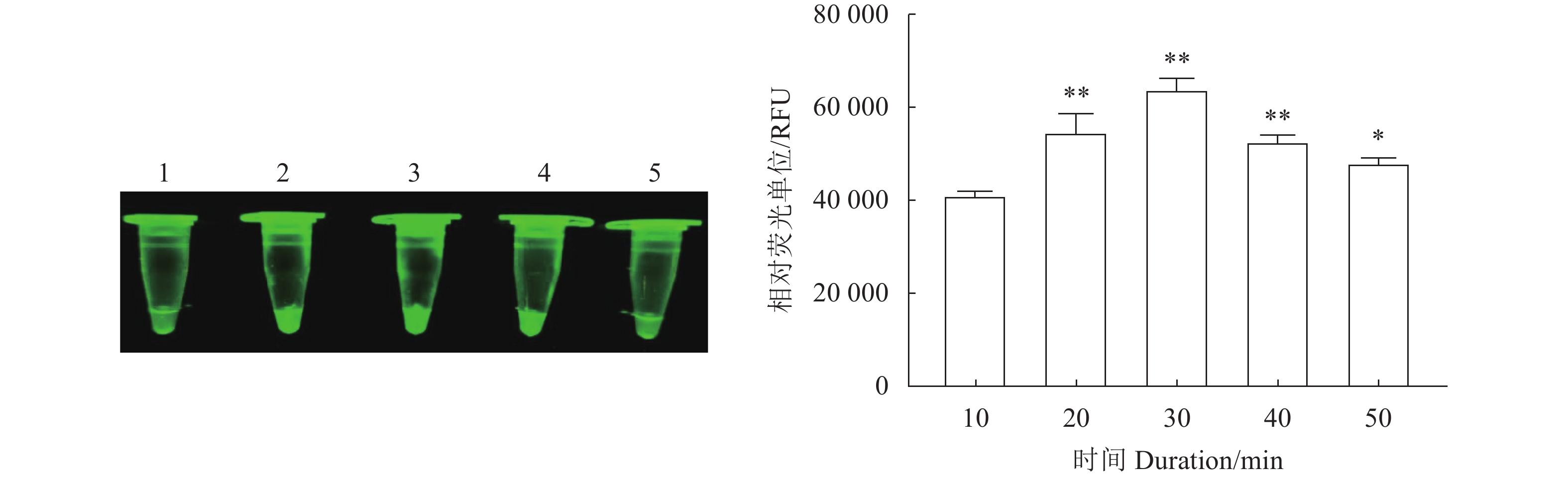
图 4 N-MDPV荧光RPA检测方法不同反应时间的扩增结果
1~5分别为10、20、30、40、50 min。*、**:与10 min相比,差异显著(P<0.05)或极显著(P<0.01)。
Figure 4. Amplifications under varied reaction time of RPA for N-MDPV
1–5: 10, 20, 30, 40, and 50m, respectively; * and **: significant difference from 10m at P<0.05 and extremely significant difference from 10m at P<0.01, respectively.
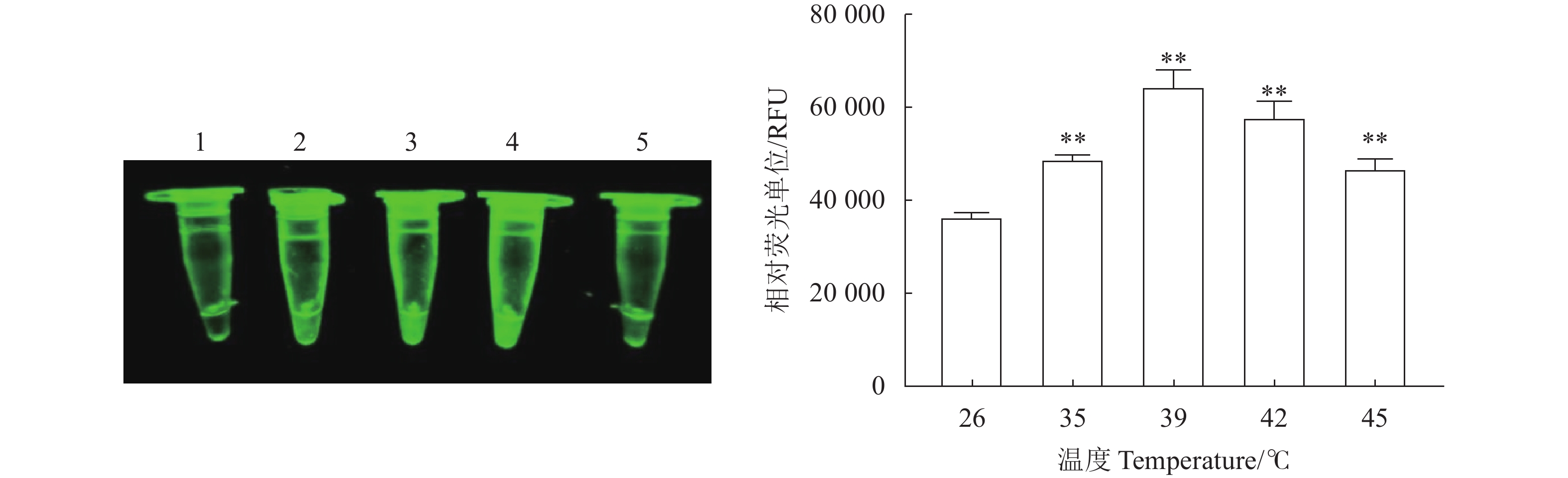
图 5 N-MDPV荧光RPA检测方法的敏感性
1:100 ng·μL−1;2:10 ng·μL−1;3:1 ng·μL−1;4:100 pg·μL−1;5:10 pg·μL−1;6:1 pg·μL−1;7:100 fg·μL−1;8:10 fg·μL−1;9:1 fg·μL−1;10:1 ag·μL−1;11:阴性对照。*、**:与阴性对照相比,差异显著(P<0.05)或极显著(P<0.01)。
Figure 5. Sensitivity of constant temperature fluorescent RPA assay
1: 100ng·μL−1; 2: 10ng·μL−1; 3: 1ng·μL−1; 4: 100pg·μL−1; 5: 10pg·μL−1; 6: 1pg·μL−1; 7: 100 fg·μL−1; 8: 10 fg·μL−1; 9: 1 fg·μL−1; 10: 1 ag·μL−1; 11: negative control; * and **: significant difference from negative control at P<0.05 and extremely significant difference from negative control at P<0.01, respectively.
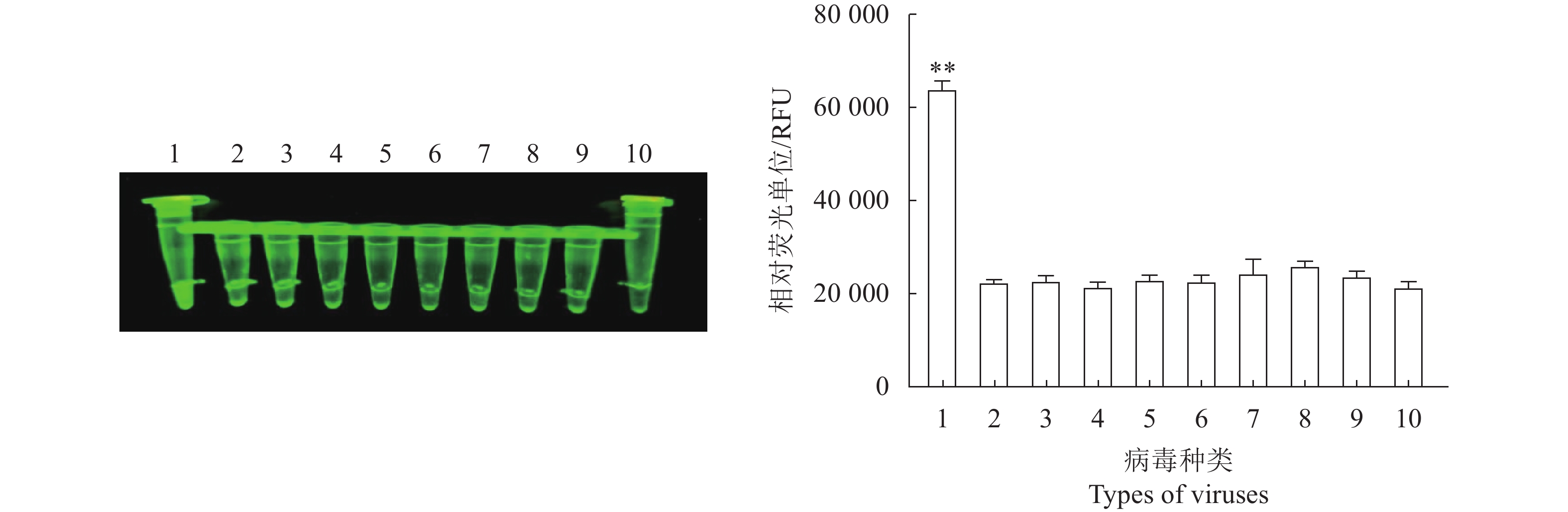
图 6 N- MDPV荧光RPA检测方法的特异性
1:新型番鸭细小病毒;2:鸭腺病毒3型;3:禽腺病毒4型;4:鸭腺病毒3型+鸭坦布苏病毒;5:鸭瘟病毒;6:鸭病毒性肝炎病毒;7:鸭圆环病毒;8:鸭坦布苏病毒;9:新型鸭呼肠孤病毒;10:阴性对照。**:与阴性对照相比,差异极显著(P<0.01)。
Figure 6. Specificity of constant temperature fluorescent RPA assay
Note: 1: N-MDPV plasmid; 2: duck adenovirus type 3; 3: duck adenovirus type 4; 4: Duck adenovirus type 3 + duck Tembousu virus; 5: duck plague virus; 6: duck viral hepatitis virus; 7: circovirus; 8: duck Tambousu virus; 9: novel duck reovirus; 10: negative control. **: extremely significant difference from negative control at P<0.01.
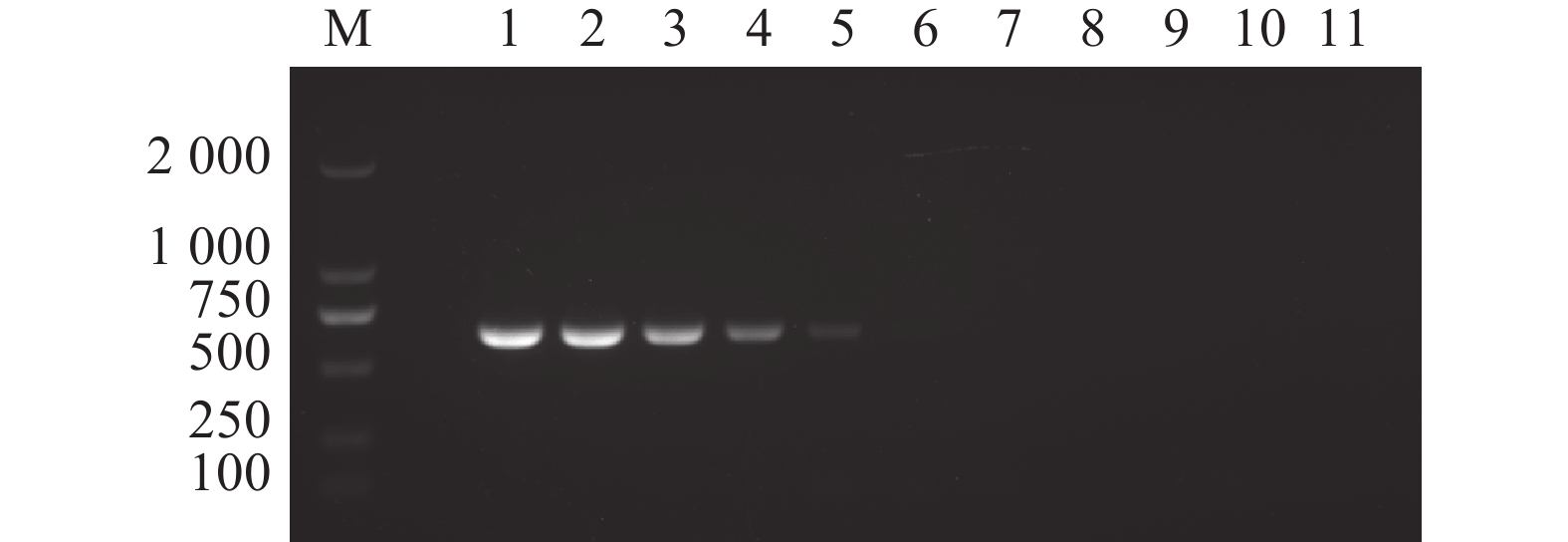
图 7 N-MDPV PCR 检测方法的敏感性
M:DL 2000 DNA分子质量标准;1:100 ng·μL−1;2:10 ng·μL−1;3:1 ng·μL−1;4:100 pg·μL−1;5:10 pg·μL−1;6:1 pg·μL−1;7:100 fg·μL−1;8:10 fg·μL−1;9:1 fg·μL−1;10:1 ag·μL−1;11:阴性对照。
Figure 7. Sensitivity of constant temperature fluorescent RPA assay
M: DL 2000 DNA marker; 1: 100ng·μL−1; 2: 10ng·μL−1; 3: 1ng·μL−1; 4: 100 pg·μL−1; 5: 10 pg·μL−1; 6: 1 pg·μL−1; 7: 100 fg·μL−1; 8: 10 fg·μL−1; 9: 1 fg·μL−1; 10: 1 ag·μL−1; 11: negative control.
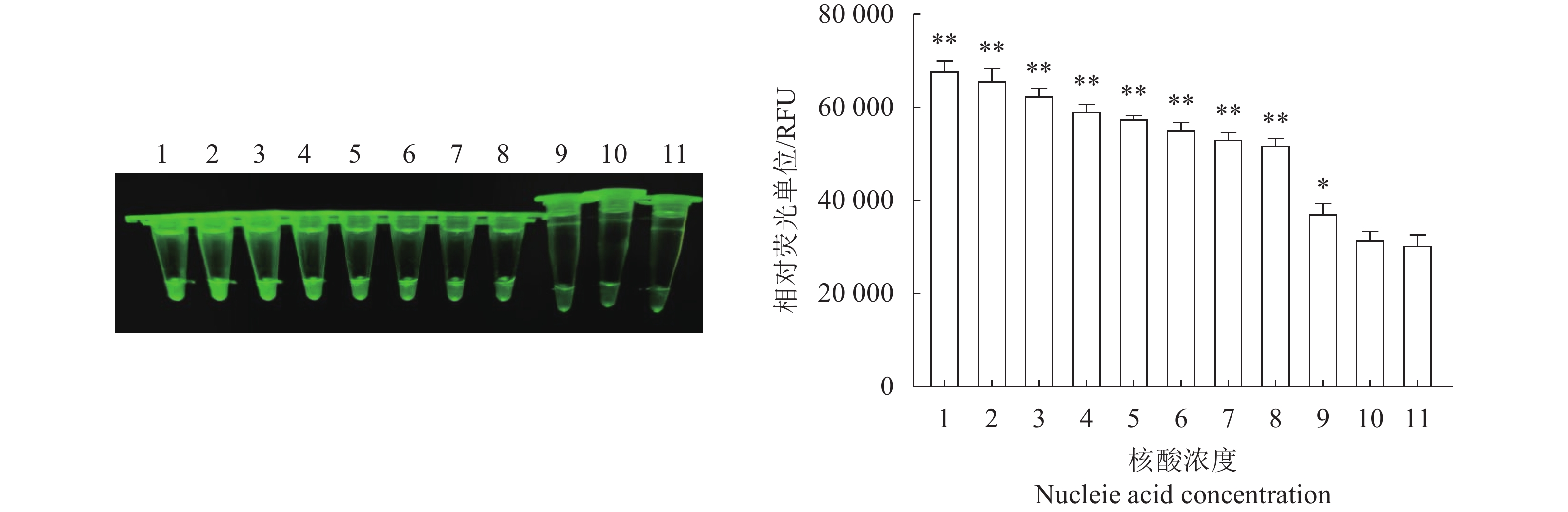
图 8 N-MDPV荧光RPA检测方法的敏感性
1:100 ng·μL−1;2:10 ng·μL−1;3:1 ng·μL−1;4:100 pg·μL−1;5:10 pg·μL−1;6:1 pg·μL−1;7:100 fg·μL−1;8:10 fg·μL−1;9:1 fg·μL−1;10:1 ag·μL−1;11:阴性对照。*、**:与阴性对照相比,差异显著(P<0.05)或极显著(P<0.01)。
Figure 8. Sensitivity of constant temperature fluorescent RPA assay
1: 100 ng·μL−1; 2: 10ng·μL−1; 3: 1 ng·μL−1; 4: 100 pg·μL−1; 5: 10 pg·μL−1; 6: 1 pg·μL−1; 7: 100 fg·μL−1; 8: 10 fg·μL−1; 9: 1 fg·μL−1; 10: 1 ag·μL−1; 11: negative control; * and **: significant difference from negative control at P<0.05 and extremely significant difference from negative control at P<0.01. respectively.
表 1 N-MDPV的RPA引物和探针
Table 1. RPA primers and probes of N-MDPV
引物和探针
Primers and probes序列(5′-3′)
Sequence(5′-3′)N-MDPV-228F AGGCGCTTATGGCACCATGGGCCGCAATTGG N-MDPV-228R CCTAAAATATTTTGGGCTGGGATGCTGGAAG N-MDPV-193F ACTCACACAGAAGCAGAGGCTTCCAGCATCC N-MDPV-193R TAGTGTTTTGTTCATTCGTTACAGTCTTGCC N-MDPV-230R CCAAGAACATCAAGATCTGAACTCGTAGGAG N-MDPV-250R AAACCATTCCTGGTAAAGCTCCAAGAACATC N-MDPV-probe 5′ACAGATCTGGCAGCACTGCAGCAGGAATAA
AFGHQATTATGGTAACGGACG -C3 Spacer表 2 N-MDPV RPA 与 PCR 方法、病毒分离法临床样本检测结果比较
Table 2. Comparison between detections by constant temperature fluorescent RPA assay and PCR on clinical samples
检测方法
Detecting
Method样品数
No. of
samples阳性样品数
No. of
positive阴性样品数
No. of
negative阳性率
Positive
rate%RPA恒温检测
RPA constant
temperature detection38 14 24 36.8 常规 PCR
Conventional PCR38 14 24 36.8 病毒分离鉴定法
Virus isolation and
identification method38 12 26 31.6 -
[1] 林世棠, 郁晓岚, 陈炳钿, 等. 一种新的雏番鸭病毒性传染病的诊断 [J]. 中国畜禽传染病, 1991, 13(2):25−26.LIN S T, YU X L, CHEN B D, et al. Diagnosis of a new viral infectious disease in Muscovy duck [J]. Chinese Journal of Preventive Veterinary Medicine, 1991, 13(2): 25−26. (in Chinese) [2] 胡奇林, 吴振兖, 周文谟, 等. 雏番鸭细小病毒病的流行病学调查 [J]. 中国兽医杂志, 1993, 29(6):7−8.HU Q L, WU Z Y, ZHOU W M, et al. Epidemiological investigation of parvovirus disease in Muscovy ducks [J]. Chinese Journal of Veterinary Medicine, 1993, 29(6): 7−8. (in Chinese) [3] 黄瑜, 万春和, 傅秋玲, 等. 新型番鸭细小病毒的发现及其感染的临床表现 [J]. 福建农业学报, 2015, 30(5):442−445. doi: 10.3969/j.issn.1008-0384.2015.05.004HUANG Y, WAN C H, FU Q L, et al. The identity and clinic infectious symptoms of the new genotype Muscovy duck parvovirus [J]. Fujian Journal of Agricultural Sciences, 2015, 30(5): 442−445. (in Chinese) doi: 10.3969/j.issn.1008-0384.2015.05.004 [4] 孟婷, 朱善元, 夏文龙, 等. 番鸭细小病毒LAMP检测方法的建立 [J]. 中国动物传染病学报, 2019, 27(1):25−29.MENG T, ZHU S Y, XIA W L, et al. Development of a loop-mediated isothermal amplification assay for detection of Muscovy duck parvovirus [J]. Chinese Journal of Animal Infectious Diseases, 2019, 27(1): 25−29. (in Chinese) [5] EULER M, WANG Y J, OTTO P, et al. Recombinase polymerase amplification assay for rapid detection of Francisella tularensis [J]. Journal of Clinical Microbiology, 2012, 50(7): 2234−2238. doi: 10.1128/JCM.06504-11 [6] 刘孟啸, 蔡姝, 刘建钗, 等. 鸭腺病毒3型荧光RPA恒温快速检测方法的建立与应用 [J]. 中国兽药杂志, 2023, 57(11):8−15.LIU M X, CAI S, LIU J C, et al. Rapid detection of duck adenovirus type 3 based on isothermal recombinase polymerase amplification assay [J]. Chinese Journal of Veterinary Drug, 2023, 57(11): 8−15. (in Chinese) [7] 袁嘉康, 李林岳, 庞俊增, 等. 猪流行性腹泻病毒RPA-LFD检测方法的建立 [J]. 中国兽医学报, 2023, 43(6):1127−1132.YUAN J K, LI L Y, PANG J Z, et al. Establishment of RPA-LFD method for detection of porcine epidemic diarrhea virus [J]. Chinese Journal of Veterinary Science, 2023, 43(6): 1127−1132. (in Chinese) [8] ABD EL WAHED A, EL-DEEB A, EL-THOLOTH M, et al. A portable reverse transcription recombinase polymerase amplification assay for rapid detection of foot-and-mouth disease virus [J]. PLoS One, 2013, 8(8): e71642. doi: 10.1371/journal.pone.0071642 [9] 王建昌, 王金凤, 刘立兵, 等. 非洲猪瘟病毒RPA等温检测方法的建立 [J]. 中国动物检疫, 2016, 33(7):78−81,94. doi: 10.3969/j.issn.1005-944X.2016.07.024WANG J C, WANG J F, LIU L B, et al. Rapid and sensitive detection of African swine fever virus by recombinase polymerase amplification [J]. China Animal Health Inspection, 2016, 33(7): 78−81,94. (in Chinese) doi: 10.3969/j.issn.1005-944X.2016.07.024 [10] DAHER R K, STEWART G, BOISSINOT M, et al. Recombinase polymerase amplification for diagnostic applications [J]. Clinical Chemistry, 2016, 62(7): 947−958. doi: 10.1373/clinchem.2015.245829 [11] 万春和, 傅秋玲, 陈翠腾, 等. 基因重组型番鸭细小病毒FJM3的全基因组特征 [J]. 中国兽医学报, 2016, 36(11):1836−1841.WAN C H, FU Q L, CHEN C T, et al. Molecular characterization of the genome for recombinant Muscovy duck parvovirus strain FJM3 [J]. Chinese Journal of Veterinary Science, 2016, 36(11): 1836−1841. (in Chinese) [12] 刘家森, 姜骞, 司昌德, 等. 番鸭细小病毒与鹅细小病毒PCR鉴别诊断方法的建立 [J]. 中国兽医科学, 2007, 37(6):469−472.LIU J S, JIANG Q, SI C D, et al. Establishment of PCR assay for differentiation of Muscovy duck parvovirus from goose parvovirus [J]. Veterinary Science in China, 2007, 37(6): 469−472. (in Chinese) -






 下载:
下载: