Effects of Corn Meal and Flaxseed Oil Dietary Supplements on Fat Content in Milk and Rumen Microbial Flora of Dairy Cows
-
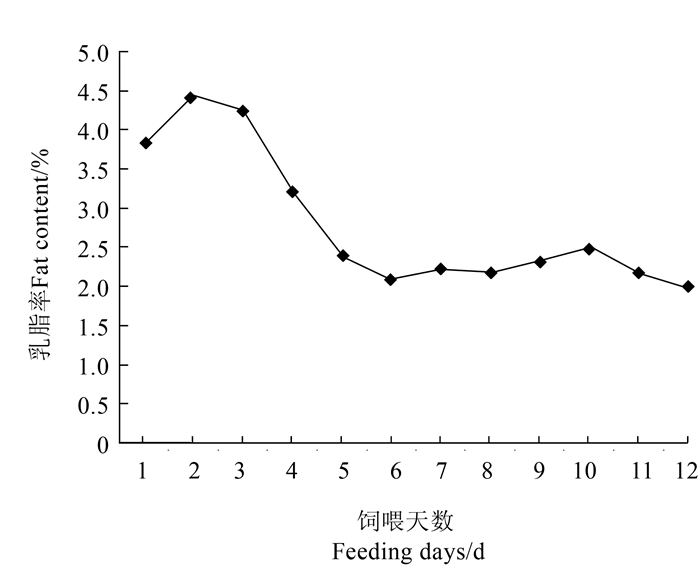
摘要:目的 阐释日粮添加玉米粉与油脂诱导奶牛低乳脂情况下,奶牛瘤胃细菌菌群的变化情况。方法 采用自身对照试验设计,选取4头体况相近、健康的泌乳期中国荷斯坦奶牛。试验开始前先以基础日粮预饲并以自身作对照。试验期奶牛日粮中添加玉米粉1.5 kg·头-1·d-1,添加胡麻油150 mL·头-1·d-1。预饲期为7 d,试验期为15 d,分析奶牛乳脂率及瘤胃细菌菌群变化情况。结果 在饲料淀粉营养水平达到27%左右水平上额外添加胡麻油150 mL·头-1·d-1,连续添加15 d使奶牛的乳脂率极显著降低(P < 0.01),乳糖显著升高(P < 0.05),成功诱导奶牛乳脂降低。瘤胃细菌研究表明,在门水平上共检测到29个菌门,与对照组相比,玉米粉和油脂诱导乳脂降低后奶牛瘤胃导软壁菌门丰度显著降低(P < 0.05),放线菌门与浮霉菌门丰度极显著增加(P < 0.01);螺旋菌门丰度显著增加(P < 0.05)。在属水平上检测到389个菌属,其中110个菌属有显著差异(P < 0.05)。与对照组相比,试验组奶牛瘤胃乳酸菌杆菌属丰度极显著增加(P < 0.01),肠球菌属丰度极显著降低(P < 0.01),克里斯滕森菌属显著降低(P < 0.05),普雷沃菌属丰度显著增加(P < 0.05),毛螺旋菌极显著降低(P < 0.01),未注释毛螺旋菌属丰度显著增加,丁酸弧菌属-2(Butyrivibrio-2)丰度显著降低(P < 0.05),瘤胃球菌属-1丰度显著提高。结论 日粮添加玉米粉与油脂诱导奶牛乳脂降低,瘤胃细菌菌群多样性未产生显著影响,但菌群结构及丰度发生了较大改变,产酸菌属与淀粉利用菌属增加显著。Abstract:Objective Changes on the fat content of milk from and rumen microbial flora in dairy cows fed with corn meal and flaxseed oil supplemented forage were studied.Method In a self-control experimentation, 4 Chinese Holstein cows of similar physical conditions and normal lactation were fed on a daily diet added with 1.5 kg of corn meal and 150 mL per animal of flaxseed oil for 7 pre-trial days followed by 15 days for the test.Result In the feed containing approximately 27% starch, the dietary supplements significantly lowered the fat content (P < 0.01) with a significantly higher lactose content (P < 0.05) in the milk produced by the cows. There were 29 microbial phyla found in the rumen. The flora of Tenericutes significantly decreased (P < 0.05), Actinobacteria and Planctomycetes significantly increased (P < 0.01), and Spirochaetae significantly increased (P < 0.05) over control. Among the 389 genera of microbes in the rumen, 110 were significantly different (P < 0.05). Comparing to control, Lactobacillus increased significantly (P < 0.01), Enterococcus decreased significantly (P < 0.01), Christensenellaceae R-7-group decreased significantly (P < 0.05), Prevotellaceae-UCG-001 increased significantly (P < 0.05), Lachnospiraceae-UCG-006 decreased significantly (P < 0.01), Lachnospiraceae-NA increased significantly, Butyrivibrio-2 decreased significantly (P < 0.05), and Ruminococcus-1 increased significantly.Conclusion Under the experimental conditions, the dietary supplementation of corn meal and flaxseed oil significantly reduced the fat content in milk of the dairy cows. It did not significantly alter the diversity but significantly changed the structure and abundance of the microbial flora in the cow rumen. The acidogenic as well as starch-utilizing bacteria significantly increased.
-
Keywords:
- dairy cow /
- milk fat reduction /
- 16S rDNA /
- high-throughput sequencing /
- rumen microbes
-
0. 引言
【研究意义】水稻光温敏核不育基因的发现与利用是选育两系杂交水稻的重要环节,利用基因编辑技术突变水稻光温敏核不育基因,对定向创制水稻光温敏核不育系具有重要意义。【前人研究进展】农垦58S是我国研究人员最早发现的一个水稻光温敏核不育系[1]。以农垦58S为不育基因供体,育种者选育了一系列光温敏核不育系应用于两系杂交稻生产[2]。张启发研究团队研究表明,pms3是决定农垦58S育性的光敏雄性核不育基因[3-4],它的转录本是一条长度为1 236 bp的长链非编码RNA,命名为长日特异性雄性不育相关RNA(long-day-specific-male-fertility-associated RNA,LDMAR)[5]。在农垦58S中,由一个C变为G的单碱基突变,使这条LDMAR的甲基化程度产生明显差异,造成长日照条件下农垦58S中的LDMAR转录量降低,促使发育中的花药提前进入程序化死亡,导致花粉不能正常发育,产生了水稻农垦58S光敏雄性核不育表型[5]。在短日条件下,水稻花粉粒的正常发育并不需要LDMAR来维持,所以农垦58S育性可以恢复[5]。庄楚雄课题组以温敏核不育系材料培矮64S为研究对象,发现ptms12-1(pms3)决定了培矮64S温敏不育特征。ptms12-1是一个非编码RNA基因,它的原始转录本在经过2次加工剪切后,最终形成了一个21 nt的小RNAosa-smR5864w[6]。水稻品种培矮64S的小RNA序列为CAU UGU UUG UCU ACC AUC CAU,而温敏不育水稻培矮64S在该小RNA序列第11个碱基处存在一个由C变为G的单碱基突变(CAU UGU UUG UGU ACC AUC CAU),形成了osa-smR5864m。在高温条件下,这个单碱基突变一方面可能影响了小RNA的表达水平,另一方面也有可能影响了小RNA与下游靶基因的结合能力而最终导致温敏雄性不育特征[6]。以上两项研究结果表明,pms3位点的同一个SNP在粳稻农垦58S中产生了光敏不育性,而在籼稻培矮64S内产生了温敏不育性,说明了水稻光温敏核不育性遗传机理的复杂性。由于osa-smR5864w是一非编码小RNA,其编码序列上的任何新改变都有可能影响其正常表达量或改变其对下游基因的调控功能,从而使突变体表现类似农垦58S的光温敏核不育特征。近年来以TALEN和CRISPR/Cas-9为代表的基因编辑技术已广泛应用于水稻基因定向突变,快速有效地获得了定向改良性状[7-12]。【本研究切入点】利用基因编辑技术对编码osa-smR5864w的序列进行定向突变,可能创制出新的光温敏核不育复等位基因,但目前尚未见到相关报道。【拟解决的关键问题】由于小RNA osa-smR5864w上的原始突变位置附近无理想的CRISPR/Cas-9编辑所需的NGG结构,因此本研究利用TALEN方法对编码该小RNA的基因组区间进行定点突变。构建了1个靶向水稻PMS3的TALEN载体,以籼稻品种明恢86和粳稻品种日本晴品种为遗传突变对象,期望通过定向突变PMS3基因,获得不同类型的小RNA osa-smR5864序列突变体,分析不同突变体的雄性育性光温反应变化规律,旨在为基于定向编辑水稻PMS3基因的方法培育水稻两系不育系材料提供科学依据。
1. 材料与方法
1.1 试验材料
粳稻品种日本晴,由福建省农业遗传工程重点实验室保存。明恢86,由三明市农业科学院提供。大肠杆菌Escherichia coli感受态DH5α和根癌农杆菌Agrobacterium tumefaciens感受态LBA4404由福建省农业遗传工程重点实验室保存。TALEN载体构建试剂盒由上海斯丹赛生物技术有限公司提供。
1.2 试验方法
1.2.1 载体构建及水稻遗传转化
选择5′-GGT GAA GCA AAG AAG-3′及5′-ACT CTT GAT GGA T-3′序列作为TALEN载体的左右臂,设计的基因编辑靶点位于小RNA osa-smR5864w之上。利用上海斯丹赛生物技术有限公司提供的TALEN载体构建试剂盒,参照说明书中的方法,构建TALEN载体。将构建好的质粒通过热激转化到DH5α大肠杆菌感受态细胞。挑取3个正常生长的单克隆培养,于铂尚生物技术(福州)有限公司测序,正向测序引物序列为305F:5′-CTC CCC TTC AGC TGG ACA C-3′,反向测序引物序列为306R:5′-AGC TGG GCC ACG ATT GAC-3′。将测序结果中的核酸序列翻译成氨基酸形式后进行比对,验证翻译的TALE蛋白是否正确,选取1个序列正确的质粒命名为TALEN-PMS3,将该质粒载体电击导入LBA4404感受态细胞中,通过农杆菌介导的方法[13]转化日本晴和明恢86的愈伤组织,获得T0代植株。
1.2.2 转基因植株鉴定
使用CTAB法[14]从水稻叶片中提取基因组DNA。以T0代各单株的基因组DNA为模板,通过上游引物HptF和下游引物HptR(表 1)PCR扩增潮霉素磷酸转移酶基因(标记基因目的条带大小为419 bp),以扩增出目的条带的为转基因阳性株。以T1和T2代各单株的基因组DNA为模板,通过HptF/HptR进行PCR扩增潮霉素磷酸转移酶基因,无目的条带的单株即为分离出的非转基因植株。
表 1 试验所用引物Table 1. Primers employed引物
Primer序列(5′- 3′)
Sequence(5′- 3′)试验目的
ApplicationHptF AAGCTGCATCATCGAAATTG 转基因阳性株检测 HptR TCGTTATGTTTATCGGCACT 305F CTCCCCTTCAGCTGGACAC TALEN质粒验证 306R AGCTGGGCCACGATTGAC LDF1 TAAGAACTGCTGCTCCAAAT PMS3基因扩增、测序 LDR1 CGGCTCCGTTATAGATAGAC 1.2.3 靶位点突变检测
以水稻PMS3基因组序列为参考,设计正向引物LDF1和反向引物LDR1(表 1),PCR扩增T0、T1和T2代各植株的PMS3基因,PCR扩增产物预计410 bp。PCR产物于铂尚生物技术公司(福州)进行测序,通过比对测序结果鉴定PMS3突变体。
1.2.4 突变体育性观察及鉴定
T2代pms3突变体及野生型对照在福建省农业科学院生物技术研究所福州寿山基地作中稻种植,明恢86来源的材料于5月20播种,日本晴来源的材料于6月20日播种,秧苗于25 d秧龄时移栽于大田,每份材料种植100株,采取正常田间管理。水稻抽穗时,摘取即将开花的小穗上、中、下各2个颖花制片,用1% I2+0.1% IK对花粉进行染色,每份材料制取10个样本,利用显微镜观察并计算花粉黑染率。套袋10个单穗,25 d后调查自交结实率。
2. 结果与分析
2.1 TALEN系统介导的PMS3序列特异性突变分析
构建了TALEN-PMS3载体,通过农杆菌介导的方法将该载体转化日本晴和明恢86的愈伤组织。共获25个转基因T0代克隆,其中日本晴获10个克隆,明恢86获15个克隆。对每个克隆靶位点序列的测序结果显示,在日本晴中有4株产生了突变,明恢86有5株产生了突变,突变率分别为40.0%和33.3%。
通过T0代植株自交,收获了上述含有靶序列突变的9株水稻T1代种子。在T1代中,通过对单株潮霉素磷酸转移酶基因及突变位点的检测,分离出非转基因纯合突变单株。各种纯合突变体序列如图 1和表 2所示。以日本晴为背景,获得了3种纯合突变类型(插入1 bp,缺失1 bp和缺失4 bp),以明恢86为背景,获得了5种纯合突变类型(插入12 bp,缺失3 bp,缺失4 bp和缺失62 bp,以及缺失82 bp外加插入23 bp)。以上突变体序列分析表明,通过TALEN方法,在日本晴和明恢86背景下都成功获得了PMS3序列的定向突变。
![]() 图 1 T1代pms3纯合突变体序列注:WT表示野生型序列; N-pms3-#和M-pms3-#分别代表日本晴和明恢86背景中的突变植株。在野生型序列中,osa-smR5864的编码序列标注为灰底字母,其中带下划线的碱基G为农垦58S的原始突变位点。黑色三角形所处位置为缺失突变发生的位置,空心三角形处为插入突变发生的位置。Figure 1. Sequencing chromatograms of mutations in T1 homozygous pms3 mutant linesNote: WT indicates wild-type sequence; N-pms3-# and M-pms3-# represent the mutant plants in Nipponbare and MH86 backgrounds, respectively. In the wild type sequence, the osa-smR5864 sequence is marked in gray letters, the underlined G is the original mutation site of Nongken 58S.The deletion and insertion sites are indicated by black triangle and hollow triangle, respectively.表 2 T1代pms3纯合突变类型Table 2. Homozygous mutation types of T1 pms3 lines
图 1 T1代pms3纯合突变体序列注:WT表示野生型序列; N-pms3-#和M-pms3-#分别代表日本晴和明恢86背景中的突变植株。在野生型序列中,osa-smR5864的编码序列标注为灰底字母,其中带下划线的碱基G为农垦58S的原始突变位点。黑色三角形所处位置为缺失突变发生的位置,空心三角形处为插入突变发生的位置。Figure 1. Sequencing chromatograms of mutations in T1 homozygous pms3 mutant linesNote: WT indicates wild-type sequence; N-pms3-# and M-pms3-# represent the mutant plants in Nipponbare and MH86 backgrounds, respectively. In the wild type sequence, the osa-smR5864 sequence is marked in gray letters, the underlined G is the original mutation site of Nongken 58S.The deletion and insertion sites are indicated by black triangle and hollow triangle, respectively.表 2 T1代pms3纯合突变类型Table 2. Homozygous mutation types of T1 pms3 lines品种背景
Background株系号
Line突变序列
Mutation sequences突变类型
Mutation types日本晴
NipponbareN-pms3-#1 ATGGATGGTAGACAA-CAATG -1 N-pms3-#2 ATGGATGGTAGA----CAATG -4 N-pms3-#3 ATTGGATGGTAGACAAACAATG +1 N-pms3-#4 ATGGATGGTAGACAA-CAATG -1 明恢86
MH86M-pms3-#1 ATGGATGGATCTAGAGTATCTGAA +12 M-pms3-#2 ATGGATGGTAGA-----------… -82 & +23 M-pms3-#3 ATGGATGGTAGAC---CAATG -3 M-pms3-#4 ATGGATGGTAGA----CAATG -4 M-pms3-#5 …--------------------TG -62 注:短划线和灰底字母分别表示缺失和插入突变,…表示已省略的序列。带下划线的G碱基为农垦58S的原始突变位点。+:插入,-:缺失,具体数字表示所涉及的核苷酸数。
Note:Deletion, insertion and omitted sequences are indicated by dashes, gray letters and dots, respectively. The underlined G is original mutation site of Nongken 58S. +: insertion, -: deletion. Data indicate number of nucleotides involved.2.2 pms3纯合突变体花粉育性正常
在福州作中稻种植时,所有日本晴和明恢86 pms3突变体及野生型亲本于8月15~20日开始抽穗。在开花期对N-pms3-#1、N-pms3-#2、N-pms3-#3、M-pms3-#1、M-pms3-#2、M-pms3-#5的T2代纯合突变体花药外型进行观察并利用碘染法对花粉的育性进行统计,结果列于表 3。分析结果显示,所有pms3突变体的花药外型正常,能够正常开裂并散粉,花粉粒经过碘-碘化钾染色后,表现为正常染色的可育花粉,突变体与野生型花粉黑染率均为90%左右,无显著差异(图 2、表 3)。而光温敏核不育系培矮64S表现为花药瘦小不开裂,花粉粒经过碘-碘化钾染色后,镜检结果显示全部为典败型花粉,无黑染花粉粒。以上结果表明,本试验通过定向突变日本晴及明恢86的PMS3基因,所获得的pms3突变体花粉在福州夏季自然长日高温条件下表现正常可育,并不能转变为不育花粉。
表 3 T2代pms3纯合突变体花粉育性及结实率Table 3. Pollen fertility and seed setting rates of T2 pms3 mutant lines材料
Lines花粉黑染率
Stained pollens rate/%每穗结实率
Seed setting rates of individual panicles/%平均结实率
Average seed setting rates/%明恢86MH86 90.3±6.2 a 95.0 86.1 93.6 94.1 89.7 78.9 83.6 82.3 76.8 91.2 87.1±6.6 a M-pms3-#1 89.9±5.1 a 88.1 92.7 91.0 89.5 85.3 90.2 77.5 81.6 80.8 85.4 86.2±5.0 a M-pms3-#2 91.5±6.1 a 91.2 89.3 90.6 88.5 79.9 91.4 87.3 82.2 78.4 86.7 86.6±4.8 a M-pms3-#5 90.5±5.8 a 90.8 83.4 84.5 78.8 91.3 92.8 76.3 87.2 80.3 87.6 85.3±5.6 a 日本晴Nipponbare 92.1±6.8 a 97.8 87.6 85.5 93.0 91.4 82.4 88.9 89.6 88.2 88.6 89.3±4.2 a N-pms3-#1 91.8±5.5 a 95.3 89.2 86.3 91.1 93.9 81.5 87.8 86.5 90.1 84.3 88.6±4.2 a N-pms3-#2 92.0±4.8 a 86.2 90.2 93.1 86.9 90.8 89 92.3 89.6 93.5 86.0 89.8±2.8 a N-pms3-#3 91.9±6.0 a 89.0 91.5 88.7 92.1 85.2 90.8 87.4 90.1 90.2 91.0 89.6±2.1 a 培矮64S P64S 0 0 0 0 0 0 0 0 0 0 0 0 注:平均花粉黑染率为10穗黑染率的平均值±标准差。同列数据后无相同小写字母表示在0.05水平差异显著(P < 0.05)。
Note:The average pollen stained rates were the mean ± standard deviation calculated from 10 panicles. The average seed setting rates were the mean ± standard deviation calculated from 10 panicles and different lowercase letters behind the number in the sane column indicated significant difterences(P < 0.05).![]() 图 2 福州自然长日高温下pms3突变体花粉育性正常注:标尺长度上4图为1 mm,下4图为100 μm。Figure 2. Anther morphology and pollen fertility of pms3 mutants showing similarities to those of wild types under natural long-day-high-temperature conditions in FuzhouNote: Scale length for the upper four graphs is 1 mm, for the lower four graphs is 100 μm.
图 2 福州自然长日高温下pms3突变体花粉育性正常注:标尺长度上4图为1 mm,下4图为100 μm。Figure 2. Anther morphology and pollen fertility of pms3 mutants showing similarities to those of wild types under natural long-day-high-temperature conditions in FuzhouNote: Scale length for the upper four graphs is 1 mm, for the lower four graphs is 100 μm.2.3 pms3纯合突变体自交结实率正常
抽穗开花期对T2代纯合突变体N-pms3-#1、N-pms3-#2、N-pms3-#3、M-pms3-#1、M-pms3-#2和M-pms3-#5进行单穗套袋,种子成熟后分单穗统计自交结实率,调查结果显示明恢86的平均结实率为(87.1±6.6)%,3个明恢86来源的pms3突变体结实率约为86%,与明恢86无显著差异,而雄性不育系培矮64S的结实率为0(表 3)。所有日本晴pms3突变体的结实率约为89%,与野生型日本晴也无显著差异。以上结实率统计结果表明,本试验通过TALEN方法虽然在日本晴和明恢86的osa-smR5864小RNA编码序列上产生了多种类型的突变,但是这些突变体在福州夏季长日高温条件下,并不能表现出类似农垦58S或培矮64S那样的光温敏雄性核不育特征。
3. 讨论与结论
诸多研究者对农垦58S的光温敏核不育基因做了大量研究,基于不同的研究材料,认为不同的水稻杂交组合中引起光敏核不育的遗传基因不同[15-17]。位于12号染色体上的pms3是形成农垦58S光敏核不育的原始突变位点[3-4]。水稻PMS3的转录产物是一条非编码RNA,它的原始转录本在经过2次加工剪切后,最终形成了一个21nt的小RNA(osa-smR5864w)[6]。该小RNA序列第11个碱基处一个由C变为G的单碱基突变,形成了osa-smR5864m,其序列为5′-CAU UGU UUG UGU ACC AUC CAU-3′,这一突变的小RNA决定了农垦58S和培矮64S的光温敏雄性不育特性[6]。
本项研究利用TALEN基因编辑技术,在粳稻品种日本晴和籼稻品种明恢86的osa-smR5864m小RNA编码序列上共产生了7种类型的插入缺失突变,有些突变类型涉及的插入或缺失碱基数较多(表 2,图 1)使osa-smR5864m序列产生了严重改变。例如,M-pms3-#5缺失了62个碱基,几乎导致编码osa-smR5864m的序列完全缺失。然而,这些突变体在福州夏季长日高温条件下,并不能表现出类似农垦58S或培矮64S那样的光温敏雄性核不育特征,这一结果与本研究的预期不符。
基于前人的研究及本试验的结果,推测有两个因素决定了这种现象。首先,可能只有原始突变处由C变为G的单碱基突变类型才能产生光温敏雄性不育表型,但因为本研究并未获得这种突变类型,所以定向突变获得的7种pms3突变体都未表现出光温敏雄性核不育特性。其次,研究者已从农垦58S中克隆出PMS1[16-17]与PMS3两个光温敏核不育基因,它们都编码21nt的小RNA,这两种小RNA在诱导水稻产生光温敏核不育性状上或许具有互作关系,当PMS1位点为野生型时,单纯突变PMS3位点可能不足以诱导形成水稻光温敏核不育表型。以上两种假设还有待进一步的实验予以解释验证。
本研究通过定向突变粳稻日本晴和籼稻明恢86的PMS3基因,使小RNA osa-smR5864的编码序列发生严重改变,但突变体并不能产生水稻光温敏雄性核不育表型,这一结果说明了PMS3位点调控水稻光温敏核不育分子机制的复杂性,具体机理有待进一步实验予以解析。
-
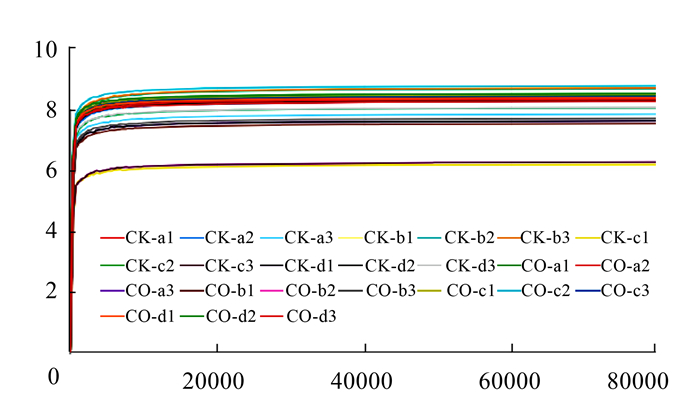
图 2 添加玉米粉和油脂对瘤胃细菌菌群Shannon指数的影响
注:a、b、c、d代表 4头不同的奶牛,每头奶牛瘤胃液样本取3次重复。横坐标表示抽取的tags数量;纵坐标表示抽取一定数量tags时所计算得到的shannon的期望值。
Figure 2. Effect of added corn meal and flaxseed oil in forage on Shannon index of rumen microbial flora
Note: a, b, c, and d represent 4 different cows. Three replicates of rumen secretion sample per cow were applied.The abscissa represents the number of extracted tags; the ordinate represents the expected value of shannon calculated when extracting a certain number of tags.
表 1 奶牛干物质基础日粮组成及营养成分
Table 1 Composition and nutrients of diet for dairy cows on dry matter base
(单位/%) 原料
Ingredient含量
Content日粮营养水平
Dietary nutrition level含量
Content豆粕 Soybean meal 11.30 粗蛋白 CP 17.14 菜籽粕 Rapeseed meal 3.20 钙 Ca 0.76 全棉籽 Whole cottonseed 3.50 磷 P 0.46 麸皮 Wheat bran 3.18 中性洗涤纤维 NDF 35.78 压片玉米 Tablet corn 11.06 淀粉 Corn 24.25 甜菜颗粒 Beet granule 2.49 产奶净能 NEL/(MJ·kg-1) 6.00 啤酒糟 Brewer's grains 3.16 苜蓿青贮 Alfalfa silage 4.12 苜蓿 Alfalfa 10.32 玉米青贮 Corn silage 45.06 碳酸氢钙 Dicalcium phosphate 0.62 石粉 Limestone 0.39 小苏打 Baking soda 0.30 氧化镁 Magnesium oxide 0.50 食盐 Salt 0.30 脱霉素 Demycin 0.10 预混料 Premix 0.40 合计 Total 100.00 注:营养水平中产奶净能为计算值,其他为实测值。
Note: NEL is calculated value; others, measured.表 2 添加玉米粉和油脂粮对奶牛乳成分的影响
Table 2 Effect of added corn meal and flaxseed oil in forage on chemical composition of cow milk
(单位/%) 项目
Item对照组
CK试验组
CO乳脂率 Milk fat 4.05±0.16A 2.10±0.06B 乳蛋白 Milk protein 3.25±0.10a 3.45±0.11a 乳糖 Lactose 4.95±0.21b 5.27±0.08a 注:同行数据肩标不同小写字母表示差异显著(P<0.05),不同大写字母表示差异极显著(P<0.01),表 4~5同。
Note: Different lowercase letters of the peer data shoulders indicate significant difference (P<0.05), and different uppercase letters indicate that the difference is extremely significant (P<0.01).The same as Table 4-5.表 3 样品在0.03距离下的Alpha丰富程度
Table 3 Alpha richness of specimens at distance 0.03
项目
ItemChao1指数
Chao1 indexAce指数
Ace indexShannon指数
Shannon indexSimpson指数
Simpson index覆盖率
Coverage/%对照组 CK 3866.47 3780.21 7.96 0.98 99.29 试验组 CO 3713.57 3642.19 8.35 0.99 99.29 P-value 0.22 0.26 0.16 0.10 - 表 4 添加玉米粉与油脂对瘤胃细菌菌群在门水平上结构的影响
Table 4 Effect of added corn meal and flaxseed oil in forage on makeup of microbial phyla in rumen
(单位/%) 门
Phylum对照组
Mean(CK)试验组
Mean(CO)拟杆菌门 Bacteroidetes 51.91±5.56 54.90±2.18 厚壁菌门 Firmicutes 35.52±2.92 37.85±1.91 变形菌门 Proteobacteria 7.58±4.04 1.34±0.07 Saccharibacteria 0.81±0.15 0.48±0.10 螺旋菌门 Spirochaetae 0.81±0.12b 1.08±0.03a 浮霉菌门 Planctomycetes 0.66±0.10B 1.23±0.18A 软壁菌门 Tenericutes 0.60±0.10a 0.34±0.06b 放线菌门 Actinobacteria 0.44±0.08B 0.99±0.17A 广古菌门 Euryarchaeota 0.18±0.04 0.09±0.03 表 5 添加玉米粉与油脂对瘤胃在属水平上菌群结构的影响
Table 5 Effect of added corn meal and flaxseed oil in forage on makeup of microbial genera in rumen
(单位/%) 属
Genus对照组
Mean(CK)试验组
Mean(CO)肠球菌属 Enterococcus 4.88±1.41A 0.24±0.08B 乳酸杆菌属 Lactobacillus 0.38±0.09B 4.56±0.58A 韦荣球菌属 Veillonellaceae-UCG-001 0.11±0.02A 0.04±0.01B 普雷沃氏菌属 Prevotellaceae-UCG-001 0.70±0.08B 1.21±0.12A 毛螺旋菌属 Lachnospiraceae-UCG-006 0.10±0.01A 0.05±0.01B 瘤胃球菌属-1 Ruminococcus-1 0.12±0.01b 0.26±0.05a 丁酸弧菌属-2 Butyrivibrio-2 0.71±0.09a 0.45±0.07b 克里斯滕森菌属 Christensenellaceae-R-7-group 4.35±0.60a 3.01±0.23b 未注释毛螺旋菌属 Lachnospiraceae-NA 0.55±0.05b 0.91±0.16a -
[1] 赵勐, 卜登攀, 张养东, 等.奶牛乳脂降低综合征理论及其分子调节机制[J].动物营养学报, 2014, 26(2):287-294. DOI: 10.3969/j.issn.1006-267x.2014.02.001 ZHAO M, BU D P, ZHANG Y D, et al.Milk fat depression in dairy cows:Theories and molecular regulation mechanism[J].Chinese Journal of Animal Nutrition, 2014, 26(2):287-294.(in Chinese) DOI: 10.3969/j.issn.1006-267x.2014.02.001
[2] 边四辈.奶牛低乳脂率的原因及解决方法探讨[J].中国奶牛, 2018(6):63-67. http://d.old.wanfangdata.com.cn/Periodical/zgnn201806018 BIAN S B. The reasons and solutions of Low-Fat-Concentration milk[J].China Dairy Cattle, 2018(6):63-67.(in Chinese) http://d.old.wanfangdata.com.cn/Periodical/zgnn201806018
[3] 王建平, 王加启, 卜登攀.精料和饱和脂肪酸对奶牛生产性能和乳中脂肪酸组成的影响[J].中国粮油学报, 2015(1):92-96. http://d.old.wanfangdata.com.cn/Periodical/zglyxb201501017 WANG J P, WANG J Q, BU D P.Effects of concentrate and supplemental saturated fatty acid on milk production and milk fatty acid profile of dairy cow[J].Journal of the Chinese Cereals and Oils Association, 2015(1):92-96.(in Chinese) http://d.old.wanfangdata.com.cn/Periodical/zglyxb201501017
[4] MA L, COOK K L, BAUMAN D E, et al. Short communication:Milk fat depression induced by conjugated linoleic acid and a high-oil and low-fiber diet occurs equally across the day in Holstein cows[J]. Journal of Dairy Science, 2015, 98(3):1851-1855. http://cn.bing.com/academic/profile?id=f855bf2f0098ba96d148cfa0a3e77db8&encoded=0&v=paper_preview&mkt=zh-cn
[5] FRUTOS P, TORAL P G, HERVÁS, G. Individual variation of the extent of milk fat depression in dairy ewes fed fish oil:Milk fatty acid profile and mRNA abundance of candidate genes involved in mammary lipogenesis[J]. Journal of Dairy Science, 2017, 100(12):1-12. http://cn.bing.com/academic/profile?id=df384e1da5556eda6af6bea8ed15bd37&encoded=0&v=paper_preview&mkt=zh-cn
[6] RAMIREZ RAMIREZ H A, CASTILLO LOPEZ E, HARVATINE K J, et al. Fat and starch as additive risk factors for milk fat depression in dairy diets containing corn dried distillers grains with soluble[J]. Journal of Dairy Science, 2015, 98(3):1903-1914. http://cn.bing.com/academic/profile?id=d33fedf82b68d4c0a4a1edfeb708ca11&encoded=0&v=paper_preview&mkt=zh-cn
[7] BELANCHE A, FUENTE G D L, PINLOCHE E, et al. Effect of diet and absence of protozoa on the rumen microbial community and on the representativeness of bacterial fractions used in the determination of microbial protein synthesis[J]. Journal of Animal Science, 2012, 90(11):3924-3936. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=77a74ff75c2409676af5d82cd3de003d
[8] GRUBB J A. Effects of an abrupt change in ration from all roughage to high concentrate upon rumen microbial numbers in sheep[J]. Appl Microbiol, 1975, 30(3):404-412. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=PubMed000002140323
[9] HUWS S A, LEE M R F, MUETZEL S M, et al. Forage type and fish oil cause shifts in rumen bacterial diversity[J]. Fems Microbiology Ecology, 2010, 73(2):396-407. http://cn.bing.com/academic/profile?id=e0d82488b4dc87557b92c8e08055deb0&encoded=0&v=paper_preview&mkt=zh-cn
[10] NETO A J, MESSANA J D, GRANJA-SALCEDO Y T, et al. Effect of starch level in supplement with or without oil source on diet and apparent digestibility, rumen fermentation and microbial population of Nellore steers grazing tropical grass[J]. Livestock Science, 2017, 202:171-179. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=1f217966deca0edd7fc0f81bfa0a5ea2
[11] WANAPAT M, MAPATO C, PILAJUN R, et al. Effects of vegetable oil supplementation on feed intake, rumen fermentation, growth performance, and carcass characteristic of growing swamp buffaloes[J]. Livestock Science, 2011, 135(1):32-37. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=8fe61274e22b693681092aef091e29f2
[12] WEIMER P J, STEVENSON D M, MERTENS D R. Shifts in bacterial community composition in the rumen of lactating dairy cows under milk fat-depressing conditions[J]. Journal of Dairy Science, 2010, 93(1):265-278. DOI: 10.3168/jds.2009-2206
[13] RICO D E, PRESTON S H, RISSER J M, et al. Rapid changes in key ruminal microbial populations during the induction of and recovery from diet-induced milk fat depression in dairy cows[J]. British Journal of Nutrition, 2015, 114(3):358-367. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=BJN114_03\BJN\BJN114_03\S0007114515001865h.xml
[14] 朱河水, 王艳玲, 杨国宇, 等.大豆黄酮对奶牛相关产奶性能的影响[J].华北农学报, 2006, 21(6):127-129. DOI: 10.3321/j.issn:1000-7091.2006.06.031 ZHU H S, WANG Y L, YANG G Y, et al.Effect of daidzein on related lactational performance of dairy cows[J]. Acta Agriculturae Boreali-Sinica, 2006, 21(6):127-129.(in Chinese) DOI: 10.3321/j.issn:1000-7091.2006.06.031
[15] SINGH K M, JISHA T K, REDDY B, et al. Microbial profiles of liquid and solid fraction associated biomaterial in buffalo rumen fed green and dry roughage diets by tagged 16S rRNA gene pyrosequencing[J]. Molecular Biology Reports, 2015, 42(1):95-103. DOI: 10.1007/s11033-014-3746-9
[16] 刘荣昌, 李英, 孙凤莉, 等.生鲜乳乳糖含量偏高问题的分析与启示[J].中国奶牛, 2014(Z1):42-44. http://d.old.wanfangdata.com.cn/Periodical/zgnn201403010 LIU R C, LI Y, SUN F L, et al.Analysis and enlightenment of the problem of high lactose content in raw milk[J]. China Dairy Cattle, , 2014(Z1):42-44.(in Chinese) http://d.old.wanfangdata.com.cn/Periodical/zgnn201403010
[17] 刘峰, 杜瑞平, 高民.不同粗饲料品质(GI)与不同精料给量水平对奶牛生产性能影响的研究[J].畜牧与饲料科学, 2012, 33(5-6):13-16. http://d.old.wanfangdata.com.cn/Periodical/nmgxmkx201205006 LIU F, DU R P, GAO M.Effects of different quality of roughage(GI) and different concentrate supply level on the production performance of dairy cows[J].Animal Husbandry and Feed Science, 2012, 33(5-6):13-16.(in Chinese) http://d.old.wanfangdata.com.cn/Periodical/nmgxmkx201205006
[18] RICO D E, HARVATINE K J. Induction of and recovery from milk fat depression occurs progressively in dairy cows switched between diets that differ in fiber and oil concentration[J]. Journal of Dairy Science, 2013, 96(10):6621-6630. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=3d232050ba569b97639b524d7594e879
[19] THANH L P, SUKSOMBAT W. Milk Yield, Composition, and Fatty Acid Profile in Dairy Cows Fed a High-concentrate Diet Blended with Oil Mixtures Rich in Polyunsaturated Fatty Acids[J].Asian-Australasian Journal of Animal Sciences, 2015, 28(6):796-806. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=86e877b0cd11c50b39d418d4ac934ac5
[20] STERK A, JOHANSSON B E O, TAWEEL H Z H, et al. Effects of forage type, forage to concentrate ratio, and crushed linseed supplementation on milk fatty acid profile in lactating dairy cows[J]. Journal of Dairy Science, 2011, 94(12):6078-6091. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=516f155cd6594d84a08314dbae2380c6
[21] GAYNOR P J, WALDO D R, CAPUCO A V, et al. Milk fat depression, the glucogenic theory, and trans-C18:1 fatty acids[J]. Journal of Dairy Science, 1995, 78(9):2008-2015. http://cn.bing.com/academic/profile?id=0d6c904358f0f32363373e356d81ddfe&encoded=0&v=paper_preview&mkt=zh-cn
[22] DHIMAN T R, NAM S H, URE A L. Factors Affecting Conjugated Linoleic Acid Content in Milk and Meat[J]. Critical Reviews in Food Science and Nutrition, 2005, 45(6):463-482. DOI: 10.1080-10408390591034463/
[23] MAIA M R G, CHAUDHARY L C, FIGUERES L, et al. Metabolism of polyunsaturated fatty acids and their toxicity to the microflora of the rumen[J]. Antonie van Leeuwenhoek, 2007, 91(4):303-314. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=235068efff326d8ed121faccd38f7e67
[24] DEVENDRA C, LEWIS D. The interaction between dietary lipids and fibre in the sheep 2. Digestibility studies[J]. Animal Production, 1974, 19(1):67-76. http://cn.bing.com/academic/profile?id=ea7f621230b06c92aad8cc259cca05f4&encoded=0&v=paper_preview&mkt=zh-cn
[25] HENDERSON C. The effects of fatty acids on pure cultures of rumen bacteria[J]. The Journal of Agricultural Science, 1973, 81(1):107-112. DOI: 10.1017/S0021859600058378
[26] 李旦.肉牛瘤胃纤维分解菌Real Time PCR定量方法的建立与应用[D].乌鲁木齐: 新疆农业大学, 2007. LI D. Development of application of a Real Time PCR approach for quantification of rumen cellulytic bacateria of cattle[D].Urumchi: Xinjiang Agricultural University, 2007.(in Chinese)
[27] HENDERSON C. A Study of the Lipase Produced by Anaerovibrio lipolytica, a Rumen Bacterium[J]. Journal of General Microbiology, 1971, 65(1):81-89. http://cn.bing.com/academic/profile?id=e6a1a2a4e2e3a43cb89b492461e78e20&encoded=0&v=paper_preview&mkt=zh-cn
[28] 李旦, 王加启, 卜登攀, 等.运用Real-time PCR方法研究日粮添加豆油与胡麻油对肉牛瘤胃纤维分解菌数量的影响[J].动物营养学报, 2008(3):256-260. DOI: 10.3969/j.issn.1006-267X.2008.03.003 LI D, WANG J Q, BU D P, et al.Determination of the effects of soybean oil and linseed oil in diets on the quantities of rumen cellulytic bacteria in beef cattle by Real-time PCR[J]. Chinese Journal of Animal Nutrition, 2008(3):256-260.(in Chinese) DOI: 10.3969/j.issn.1006-267X.2008.03.003
[29] 张迪.慢性瘤胃酸中毒对瘤胃发酵功能及乳酸代谢菌的影响[D].呼和浩特: 内蒙古农业大学, 2008. http://www.wanfangdata.com.cn/details/detail.do?_type=degree&id=Y1307648 ZHANG D. Effect of subacute rumen acidosis on rumen fermentation and populations of lactate metabolism bacteria[D].Hohhot: Inner Mongolia Agricultural University, 2008.(in Chinese) http://www.wanfangdata.com.cn/details/detail.do?_type=degree&id=Y1307648
[30] KREHBIEL C R, STOCK R A, HEROLD D W, et al. Feeding wet corn gluten feed to reduce subacute acidosis in cattle[J]. Journal of Animal Science, 1995, 73(10):2931. DOI: 10.2527/1995.73102931x
[31] LETTAT A, NOZIōRE, P, SILBERBERG M, et al. Experimental feed induction of ruminal lactic, propionic, or butyric acidosis in sheep1[J]. Journal of Animal Science, 2010, 88(9):3041-3046. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=ab55c2cc7e7fb85e3cff3d62e3f7d543
[32] GRIINARI J M, CORL B A, LACY S H. Conjugated linoleic acid is synthesized endogenously in lactating dairy cows by delta(9)-desaturase[J]. Journal of Nutrition, 2000, 130(9):2285. http://med.wanfangdata.com.cn/Paper/Detail/PeriodicalPaper_PM10958825
[33] 马涛, 刁其玉.瘤胃微生物与饲粮脂肪酸间的相互作用[J].动物营养学报, 2018, 30(5):1611-1618. DOI: 10.3969/j.issn.1006-267x.2018.05.001 MA T, DIAO Q Y.Interaction between ruminal microbes and dietary fatty acids[J]. Chinese Journal of Animal Nutrition, 2018, 30(5):1611-1618.(in Chinese) DOI: 10.3969/j.issn.1006-267x.2018.05.001
[34] HENDERSON G, COX F, GANESH S, et al. Rumen microbial community composition varies with diet and host, but a core microbiome is found across a wide geographical range[J]. Scientific Reports, 2015, 5(1):14567. http://cn.bing.com/academic/profile?id=a383a82abb2cd55c255684cd4f9fff6e&encoded=0&v=paper_preview&mkt=zh-cn
[35] TORAL P G, BELENGUER A, SHINGFIELD K J, et al. Fatty acid composition and bacterial community changes in the rumen fluid of lactating sheep fed sunflower oil plus incremental levels of marine algae[J]. Journal of Dairy Science, 2012, 95(2):794-806. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=812debad93df55cf7c8c0f1b8b6301e6
[36] 杨舒黎.日粮添加豆油和胡麻油对奶牛瘤胃细菌及发酵参数的影响[D].北京: 中国农业科学院, 2007. http://www.wanfangdata.com.cn/details/detail.do?_type=degree&id=Y1057276 YANG S L. Effect of soybean oil and linseed oil supplementation on population of ruminal bacteria and fermentation parameters in dairy cows[D].Beijing: Chinese Academy of Agricultural Sciences, 2007.(in Chinese) http://www.wanfangdata.com.cn/details/detail.do?_type=degree&id=Y1057276
[37] 姜雅慧, 卜登攀, 杨红建, 等.不饱和脂肪酸在瘤胃氢化的微生物学机制研究进展[J].华北农学报, 2015, 30(S1):376-382. DOI: 10.7668/hbnxb.2015.S1.068 JIANG Y H, BU D P, YANG H J, et al.The advance research of the microbial biohydrogenation mechanism of unsaturated fatty acid in rumen[J]. Acta Agriculturae Boreali-Sinica, 2015, 30(S1):376-382.(in Chinese) DOI: 10.7668/hbnxb.2015.S1.068
[38] 周敏, 叶子弘, 蒋林树.瘤胃氢化多不饱和脂肪酸的影响因素[J].中国农学通报, 2010, 26(8):38-44. http://d.old.wanfangdata.com.cn/Periodical/zgnxtb201008009 ZHOU M, YE Z H, JIANG L S.Study of the influencing factor of PUFA living things hydrogenation in rumen[J].Chinese Agricultural Science Bulletin, 2010, 26(8):38-44.(in Chinese) http://d.old.wanfangdata.com.cn/Periodical/zgnxtb201008009
-
期刊类型引用(3)
1. 杨大兵,胡亮,杜雪树,万丙良,夏明元,戚华雄,李进波. CRISPR/Cas9基因编辑技术创制水稻雄性不育系的研究进展. 中国农业科技导报(中英文). 2025(03): 24-34 .  百度学术
百度学术
2. 唐杰,龙湍,吴春瑜,李新鹏,曾翔,吴永忠,黄培劲. 水稻光温敏雄性不育突变体tms3650的鉴定和基因定位. 中国水稻科学. 2023(01): 45-54 .  百度学术
百度学术
3. 谭瑗瑗,汪庆,富昊伟,张渭章,吴三玲,舒庆尧. 利用花培与辐照诱变培育粳型两系不育系江79S. 核农学报. 2022(06): 1073-1079 .  百度学术
百度学术
其他类型引用(3)




 下载:
下载: