Effects of Arbuscular Mycorrhizal Fungi Inoculation on Salt-tolerance of Tomato Plants
-
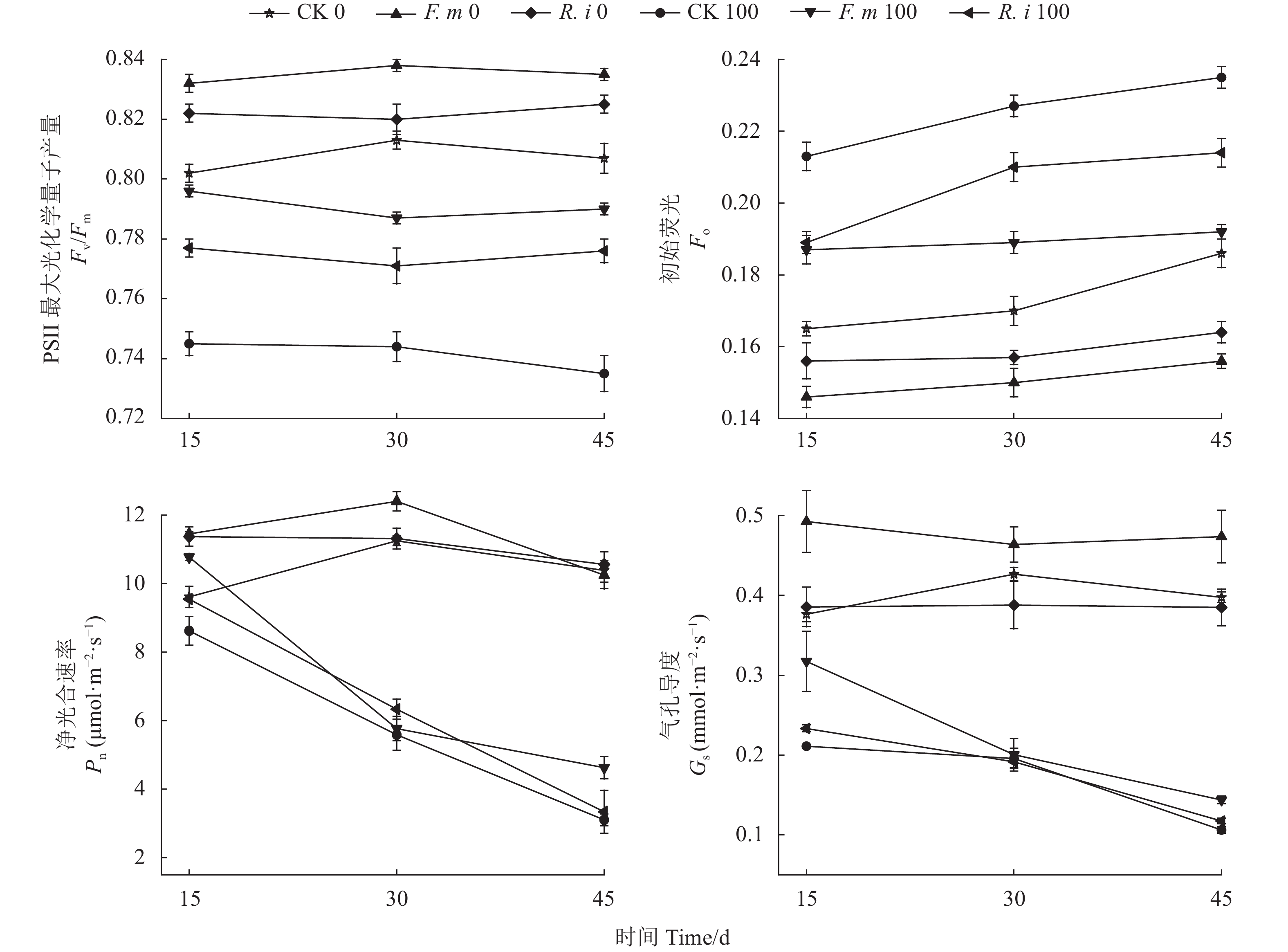
摘要:目的 研究不同丛枝菌根真菌(AMF)对番茄植株生长和抗盐胁迫效应,探究筛选出能够延缓盐分对番茄生理活性的抑制,提升植株光合碳同化能力和耐盐性能力的最佳AMF真菌。方法 针对土壤盐渍化对番茄的不良效应,通过土培法对番茄植株进行不同盐浓度(0、100 mmol·L−1)处理,经初筛得到摩西(F.m)、根内(R.i)两种丛枝菌根真菌作为接种菌剂,从生理及光合2个角度探究对盐胁迫下接种不同AMF侵染对番茄的生长状况的影响。结果 盐胁迫下番茄抗氧化酶系活性,包括超氧化物歧化酶(SOD)、过氧化物酶(POD)、过氧化氢酶(CAT)、丙二醛(MDA)含量及脯氨酸(Pro)含量分别提高了47.4%、32.9%、35.7%、61.8%、6%。菌根侵染率、光合强度降低了27.8%和54.6%。接种AMF能有效增强宿主抗盐性,其中F.m接种后番茄的脯氨酸含量降低60.7%,降低幅度是接种R.i处理的2.2倍。F.m接种后番茄的净光合速率(Pn)、气孔导度(Gs)分别提高49.1%、35.4%,增幅是接种R.i处理的1.4倍。同时,接种AMF能减轻盐毒对光合关键酶的损伤,其中接种F.m后番茄RuBP羧化酶最大增长率可达31.2%,是接种R.i真菌处理的1.1倍。结论 AMF可延缓盐分对番茄生理活性的抑制,提升植株光合碳同化能力和耐盐性,其中接种摩西(F.m)真菌对植物促生作用显著优于根内(R.i)真菌。Abstract:Objective Effect of introducing various arbuscular mycorrhizal fungi (AMF) in soil on the growth and salt-tolerance of tomato plants was studied.Method After a preliminary screening, two potentially applicable AMF, Moses (F.m) and rhizosphere (R.i), were added to potting soils with salt concentrations of 0 (CK) and 100 mmol·L−1 for a tomato plant cultivation experimentation. Physiological and photosynthetic properties of the plants were monitored.Result The AMF-treatments increased superoxide dismutase (SOD) activity by 47.4%, peroxidase (POD) activity by 32.9%, catalase (CAT) activity by 35.7%, malondialdehyde (MDA) content by 61.8%, and proline (PRO) content by 6% of the tomato plants under the imposed salt stress. Meanwhile, the mycorrhizal infection rate and photosynthetic intensity decreased by 27.8% and 54.6%, respectively. The inoculation effectively enhanced the resistance of the host plants to high salinity. The proline content of the tomato plants grown on the F.m-inoculated soil declined 60.7%, which was 2.2 times of that on R.i-inoculated counterpart. The net photosynthetic rate (Pn) and stomatal conductance (Gs) of the tomato plants on the F.m-inoculated soil increased by 49.1% and 35.4%, respectively, which were 1.4 times of R.i-inoculation. In addition, the damage to the key photosynthesis enzymes was reduced, as the maximum increase rate of RuBP carboxylase in the tomato plants rose to 31.2% under the F.m-treatment and 1.1 times of which under R.i.Conclusion The introduction of AMF in soil could delay the adverse effect of high salinity on the physiological activities, improve the photosynthetic carbon assimilation and salt tolerance, and promote the growth of tomato plants. Of the two candidates, F.m was shown to significantly superior to R.i for the application.
-
Keywords:
- Arbuscular mycorrhizal fungi /
- tomato /
- infection rate /
- physiological activity /
- salt stress
-
0. 引 言
【研究意义】百合叶烧病是一种生理性病害,国内外百合种植过程中普遍发生,严重影响切花百合和盆栽百合生产质量。其发病症状表现为百合生长至30~40 cm,花蕾出现前,顶部6~10片新叶离叶尖约2 cm处出现灰白色病斑,后期逐渐向叶尖蔓延,最后变成焦枯状。百合叶烧病具有显著的品种特异性,但目前尚未发现与叶烧病存在紧密联系的品种特征[1],因此探索百合叶烧病发病机理,对百合新种质创制和栽培技术创新具有重要意义。【前人研究进展】前人研究发现,通过剪掉一半刚展开的下部叶片[2]、在发病之前喷施氯化钙和硝酸钙溶液[3]、通过手动展开尚未展开的新叶和降低栽培环境的湿度[4]等方法能够降低叶烧病的发生率,同时施用生根剂、微生物菌肥和使用合适的栽培基质均能有效地降低叶烧病的发生[5-7]。【本研究切入点】目前,百合叶烧病发病原因及防治方法等相关研究已较为深入,其中由缺钙导致叶烧病发生已被确认,但由于钙参与多项植物生理调节过程,如何确定叶烧病发生分子机理,以及缺钙导致哪些代谢途径受阻从而产生叶烧病,是今后选育抗叶烧病品种的重要研究基础。【拟解决的关键问题】通过扫描电镜和投射电镜观察东方百合Tarrango正常叶片、轻度叶烧叶片和重度叶烧叶片超微结构的差异,并通过比较东方百合Tarrango正常叶片、叶烧叶片、正常叶片喷钙和叶烧叶片喷钙4种处理的转录组测序数据,以期探索与叶烧病发病相关的分子机理和关键调控基因。
1. 材料与方法
1.1 试验材料
以荷兰进口的Tarrango百合种球为试验材料,种植于连云港市农业科学院东辛农场实验基地日光温室,Tarrango百合生长至现蕾期,对部分百合叶烧叶片和正常叶片喷施30 mmol·L−1 硝酸钙叶面肥一次,其余百合喷施等量清水。24 h后分别取正常叶片(TarCK)、叶烧叶片(TNCK)、叶面喷钙正常叶片(TarCa)和叶面喷钙叶烧叶片(TNCa)4个处理,干冰冷藏送样检测,每个处理生物学重复3次。
1.2 转录组测序及分析试验方法
1.2.1 RNA提取及处理
上述4个处理中,取每个处理叶片混合样品1 g,参照TaKaRA全RNA提取试剂操作说明进行叶片总RNA提取。
1.2.2 文库构建及测序
百合叶片cDNA文库构建及测序均委托苏州金唯智生物科技有限公司完成。
1.2.3 转录组数据组装及基因功能注释
百合叶片转录组测序及对获得的数据库 Unigene 的全面分析和注释,均委托苏州金唯智生物科技有限公司完成。
1.3 百合叶片扫面电镜及透射电镜观察
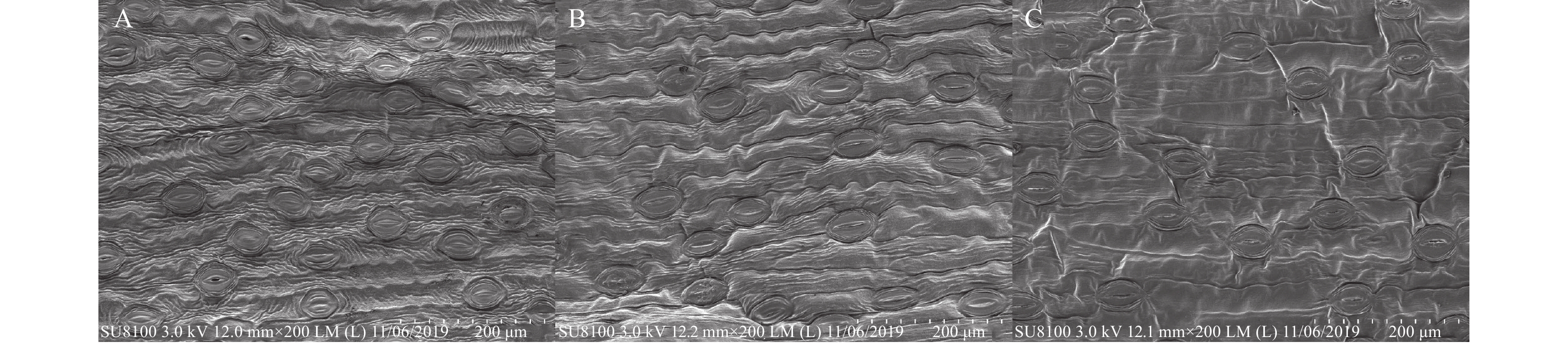
采集Tarrango正常叶片、轻度发病叶烧叶片和重度发病叶烧叶片(图1),无菌水漂洗干净后,手术刀裁成3 mm×3 mm大小,置于电镜固定液中固定2 h,再转移至4 ℃保存,4 ℃冰袋运输送样至武汉赛维尔生物科技有限公司。
2. 结果与分析
2.1 百合叶片叶烧病扫描电镜分析
如图2所示,近轴面表皮层均无气孔分布,其中Tarrango正常叶片和Tarrango轻微叶烧叶片的上表皮细胞横直径为500 μm。而Tarrango重度叶烧叶片上表皮细胞发生严重皱缩,其上皮细胞的横直径只有312.5 μm。轻度叶烧时,百合叶片上表皮细胞出现轻微的收缩,重度叶烧时,百合叶片表皮细胞表面出现褶皱痕迹。
如图3所示,百合叶片远轴面表皮层分布了大量气孔,气孔数量最多的是Tarrango重度叶烧叶片(图3-A),其次为Tarrango轻度叶烧叶片(图3-B)和Tarrango正常叶烧叶片(图3-C)叶片。Tarrango正常叶片、Tarrango轻微叶烧叶片和Tarrango重度叶烧叶片气孔直径大小基本一致,均在400 μm左右。Tarrango轻度叶烧叶片下表皮层便发生皱缩现象,重度叶烧叶片皱缩程度更加严重。
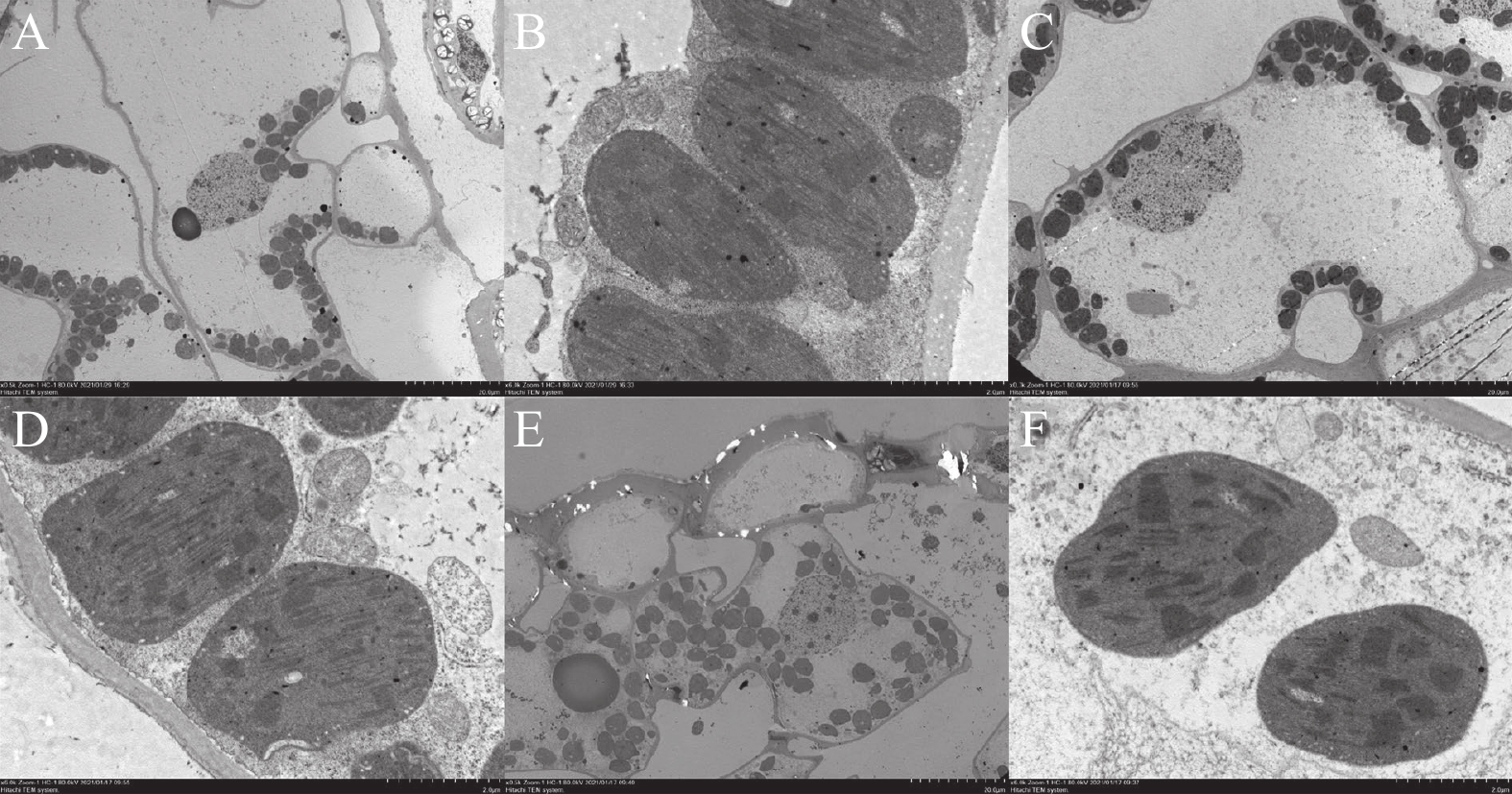
2.2 百合叶片透射电镜分析
正常叶片叶肉细胞(图4-A、B)和轻度叶烧叶肉细胞(图4-C、D)液泡较大,叶绿体分布在边缘。重度叶烧叶片的叶肉细胞由于液泡失水,叶绿体分布在整个细胞,细胞较为规则;重度叶烧细胞由于失水,整个细胞皱缩,形状不规则(图4-E、F)。在叶烧病发展过程中,叶绿体结构未发生太大变化,基粒垛叠层数较多且排列规律,3个处理中叶绿体均含有数量不等的淀粉粒。但重度叶烧叶片叶肉细胞中,线粒体数量明显较少。
2.3 转录组测序组装及unigene注释分析
使用软件Cutadapt对测序原始数据(Pass filter data)去除接头以及低质量序列,获得后续信息分析用的过滤数据(Clean data)。4个处理12个文库产生了541 393 662条序列,约80.68 Gb转录组数据,Unigene平均长度在148.63 ~149.00 bp,GC含量变化范围为49.17%~52.32%(表1)。由表2可以看出,转录组数据经组装产生了19 585 575个重叠群,349537条unigenes,平均长度为513.25 bp。在所有unigenes序列中,序列长度小于500 bp的占75.92%(表3)。Unigene注释分析显示有124 405 unigenes获得注释,占unigenes总数的35.59%,其中获得注释较多的数据库分别是Nr注释121 501(34.76%)、COG注释49 594条(14.19%)、Swissport注释72 421条(20.72%)和KEGG注释17 051条(4.88%)。
表 1 12个cDNA文库的过滤数据Table 1. Clean data of 12 cDNA library样品
Sample平均长度
Average length/bp总序列数
The total number of sequences总碱基数
Total base number /bpQ20含量
Q20 content /%Q30含量
Q30 content /%GC含量
GC /%TarCK1 148.70 46691744 6943031355 98.11 94.32 50.55 TarCK2 148.77 49717384 7396527911 98.26 94.67 49.80 TarCK3 148.63 40433794 6009572727 98.15 94.35 52.27 TarCa1 148.74 47758784 7103493445 98.15 94.44 51.41 TarCa2 148.87 40229314 5988834979 98.07 94.30 52.04 TarCa3 149.00 46500744 6928790175 98.07 94.28 51.59 TNCK1 148.98 43959714 6549244364 98.15 94.42 50.78 TNCK2 148.79 40479744 6022881697 98.23 94.59 49.17 TNCK3 148.87 45992514 6846847661 98.08 94.21 49.91 TNCa1 149.00 44762836 6669452846 98.10 94.41 52.32 TNCa2 148.96 46342564 6903233715 98.04 94.15 51.03 TNCa3 148.89 48524526 7224858395 98.09 94.31 52.03 合计 Total 541393662 80686769270 表 2 转录组序列组装分析Table 2. Summary of transcriptome assembly序列类型
Sequence type重叠群
Contig序列
Unigene最短序列长度 Minimum sequence Length/bp 25 201 最长序列长度 Maximum sequence length/bp 15 734 11 377 序列平均长度 Mean sequence length/bp 53.76 513.25 N50长度 N50 length/bp 48 686 (A+T)/% 51.94 55.72 (C+G)/% 48.06 44.28 序列总数 The total number of sequences 19 585 575 349 537 总碱基数量 Total base number/bp 1 052 959 813 179 400 360 表 3 Unigene 的长度及数量统计Table 3. Unigene length and quantity statistics长度
Length/bp数量
Number比例
Percentage/%<200 0 0.00 200~500 265 367 75.92 500~1 000 44113 12.62 1 000~1 500 17 752 5.08 1 500~2 000 10 495 3.00 ≥2 000 11 809 3.38 总数 Total 349 537 100 2.4 差异表达基因筛选及分析
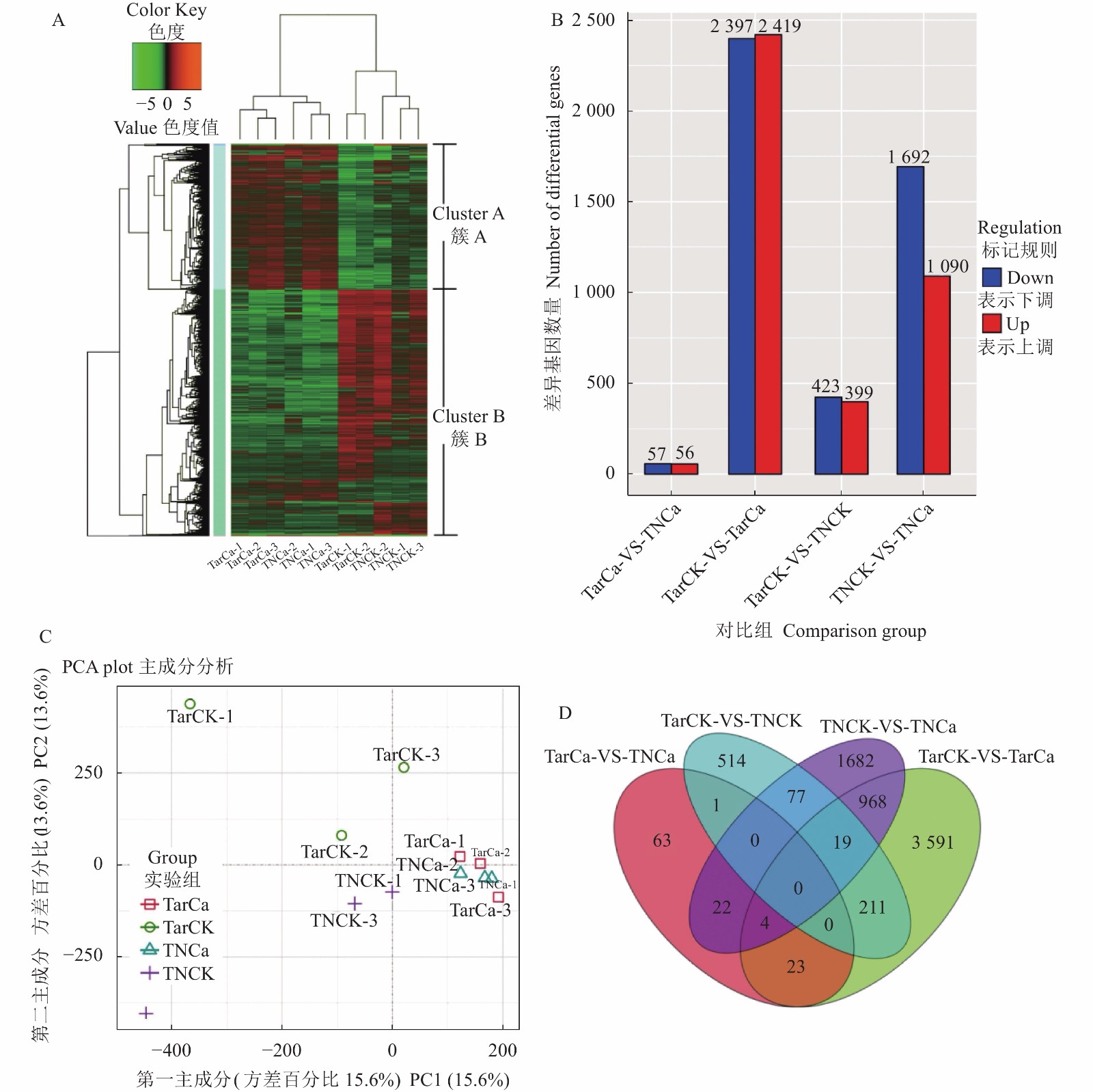
对4组处理转录组数据进行层次聚类(Hierarchical clustering)分析,喷钙2个处理TarCa与TNCa基因表达模式相近,并且与喷清水2个处理TarCK与TNCK基因表达模式存在显著区别,通过基因表达量可以将差异表达基因划分成A、B两个簇(图5-A),同时通过对转录组数据分组对比分析发现,TarCK/TarCa比较中差异表达基因最多,总数为4 816个,其中上调表达基因2 419个,下调表达基因2 397个;其次TNCK/TNCa比较中差异表达基因2 782个,其中上调表达基因1 090个,下调表达基因1 692个;TarCK/TNCK比较中差异表达基因822个,其中上调表达基因399个,下调表达基因423个;TarCa/TNCa比较中差异表达基因最少,总数为113个,其中上调表达基因56个,下调表达基因57个(图5-B)。
![]() 图 5 差异表达基因的表达谱分析注:A,差异表达基因的聚类分析;B,差异表达基因的数量;C,差异表达基因的主成分分析;D,差异基因维恩图分析。Figure 5. Expression profiling of differentially expressed genesNote: A: Cluster analysis on differentially expressed genes; B: Number of differentially expressed genes; C: Principal component analysis on differentially expressed genes; D: Venn diagram of differential genes.
图 5 差异表达基因的表达谱分析注:A,差异表达基因的聚类分析;B,差异表达基因的数量;C,差异表达基因的主成分分析;D,差异基因维恩图分析。Figure 5. Expression profiling of differentially expressed genesNote: A: Cluster analysis on differentially expressed genes; B: Number of differentially expressed genes; C: Principal component analysis on differentially expressed genes; D: Venn diagram of differential genes.通过PCA(Principal Component Analysis)分析发现,TarCa与TNCa两个处理的重复组间的变异较小,并且两个处理间的差异性也较小,而TarCK与TNCK两个处理间不但处理间的差异性较大,重复组间的变异也比较大(图5-C)。同时,在4组对照分析中,发现了特异的和共同的差异表达基因,共发现7 185个差异表达基因,包括5 860个特异差异表达基因和1 325个共同差异表达基因(图5-D)。其中TarCK/TarCa与TNCK/TNCa共差异表达基因最多,为991个;其次为TarCK/TNCK与TarCK/TarCa共差异表达基因为230个和TNCK/TNCa与TarCK/TNCK共差异表达基因为96个,TarCa/TNCa与TarCK/TNCK共差异表达基因最少,只有1个,通过上述数据表明,喷钙处理能显著降低叶烧叶片与正常叶片的差异表达基因数量。
2.5 差异基因GO富集分析
通过GO富集分析对差异表达基因进行生物学功能研究,在错误发现率FDR<0.01时,25个GO条目富集在TarCa/TNCa,25个GO条目富集在TarCK/TNCK,31个GO条目富集在TarCK/TarCa,31个GO条目富集在TNCK/TNCa。有18个GO条目在4组对比分析中均有富集,其中,“催化活性” “结合”和“代谢过程”是富集基因最多的GO条目。在4组对比分析中有5个特异性GO条目,其中“多细胞生物过程”和“繁殖”GO条目仅在TarCK/TarCa中富集,“突触部分”GO条目仅在TNCK/TNCa中富集,“细胞外基质”和“细胞外基质成分”GO条目仅在TarCa/TNCa中富集,TarCK/TNCK无特异性GO条目(图6)。
![]() 图 6 差异表达基因的GO富集分析注:1,催化活性;2,结合;3,转运活性;4,结构分子活性;5,电子载体;6,核酸结合转录因子活性;7,酶调节活性;8,抗氧化活性;9,分子传感器活性;10,细胞组分;11,细胞器;12,膜部分;13,细胞器部分;14,细胞膜;15,高分子复合物;16,胞外区;17,类核;18,细胞连接;19,代谢过程;20,细胞过程;21,单一生物过程;22,生物调节;23,应激反应;24,定位;25,发育过程;26,多生物过程;27,免疫系统过程;28,组织细胞组成或生物起源;29,生殖过程;30,多细胞生物过程;31,繁殖;32,细胞外区域部分;33,细胞外基质;34,细胞外基质成分;35,运动;36,生长;37,突触部分。Figure 6. GO enrichment analysis on differentially expressed genesNote: 1: catalytic activity; 2: binding; 3: transporter activity; 4: structural molecule activity; 5: electron carrier activity; 6: nucleic acid binding transcription factor activity; 7: enzyme regulator activity; 8: antioxidant activity; 9: molecular transducer activity; 10: cell part; 11: organelle; 12: membrane part;13: organelle part; 14: membrane; 15: macromolecular complex; 16: extracellular region; 17: nucleoid; 18: cell junction; 19: metabolic process; 20: cellular process; 21: single-organism process; 22: biological regulation; 23: response to stimulus; 24: localization; 25: developmental process; 26: multi-organism process; 27: immune system process; 28: cellular component organization or biogenesis; 29: reproductive process; 30: multicellular organismal process; 31: reproduction; 32: extracellular region part; 33: extracellular matrix; 34: extracellular matrix component; 35: locomotion; 36: growth; 37: synapse part.
图 6 差异表达基因的GO富集分析注:1,催化活性;2,结合;3,转运活性;4,结构分子活性;5,电子载体;6,核酸结合转录因子活性;7,酶调节活性;8,抗氧化活性;9,分子传感器活性;10,细胞组分;11,细胞器;12,膜部分;13,细胞器部分;14,细胞膜;15,高分子复合物;16,胞外区;17,类核;18,细胞连接;19,代谢过程;20,细胞过程;21,单一生物过程;22,生物调节;23,应激反应;24,定位;25,发育过程;26,多生物过程;27,免疫系统过程;28,组织细胞组成或生物起源;29,生殖过程;30,多细胞生物过程;31,繁殖;32,细胞外区域部分;33,细胞外基质;34,细胞外基质成分;35,运动;36,生长;37,突触部分。Figure 6. GO enrichment analysis on differentially expressed genesNote: 1: catalytic activity; 2: binding; 3: transporter activity; 4: structural molecule activity; 5: electron carrier activity; 6: nucleic acid binding transcription factor activity; 7: enzyme regulator activity; 8: antioxidant activity; 9: molecular transducer activity; 10: cell part; 11: organelle; 12: membrane part;13: organelle part; 14: membrane; 15: macromolecular complex; 16: extracellular region; 17: nucleoid; 18: cell junction; 19: metabolic process; 20: cellular process; 21: single-organism process; 22: biological regulation; 23: response to stimulus; 24: localization; 25: developmental process; 26: multi-organism process; 27: immune system process; 28: cellular component organization or biogenesis; 29: reproductive process; 30: multicellular organismal process; 31: reproduction; 32: extracellular region part; 33: extracellular matrix; 34: extracellular matrix component; 35: locomotion; 36: growth; 37: synapse part.2.6 差异基因KEGG富集分析
在植物体内,Ca2+参与的代谢调控十分复杂,通过KEGG代谢通路分析,在本次转录组测序中“剪接体” “代谢途径” “丙酮酸代谢” “次生代谢产物的生物合成”和“光合作用生物的碳固定作用”代谢途径在4组试验处理对比分析中都有基因富集,经过差异基因COG分析,4组试验处理对比分析共有25个COG分组,其中4组试验处理共有COG功能分类22个,包括“细胞外结构” “信号转导机制” “未知功能基因” “细胞内运输、分泌和囊泡运输” “翻译、核糖体结构和生物发生” “细胞壁/膜/信封生源论” “一般功能预测基因” “复制、重组和修复” “碳水化合物运输和代谢” “无机离子的转运和代谢” “细胞骨架” “氨基酸的运输和代谢” “脂质运输与代谢” “细胞周期控制,细胞分裂,染色体分裂” “能源生产与转换” “染色质结构和动力学” “翻译后修饰,蛋白质转换,伴侣” “辅酶运输和代谢” “RNA加工和修饰” “次级代谢产物生物合成、运输和分解代谢”。
2.7 显著差异基因分析
结合扫描电镜和透射电镜分析,本研究重点关注叶烧病发病过程和喷施钙处理中,细胞膜通透性相关基因的表达变化。如表4所示,在百合叶烧病发病过程中即TarCK/TNCK对比分析中,显著下调基因有参与脱落酸信号的负调控的FOLK基因(Farnesol kinase 法呢醇激酶)[8]、影响脂类物质生物合成和细胞膜稳定性的PLD1_2基因(phospholipase D1/2 磷脂酶D1/2)[9]、ATPeF1B基因(F-type H+-transporting ATPase subunit bet膜上ATP合酶)和KCS基因(3-ketoacyl-CoA synthase 3-酮脂酰辅酶A合酶)[10]。同时CALM基因(Calmodulin 钙调蛋白)[11]、ENO基因(enolase 烯醇酶)[12]和pel基因(Pectate lyase 果胶酸裂解酶)[13]等响应钙离子信号和影响木质素和果胶生物合成的基因下调。在百合叶烧病发病过程中表达量上调的基因有促进生成脱落酸的AAO基因(abscisic-aldehyde oxidase 脱落醛氧化酶)[14]。
表 4 显著差异表达基因Table 4. Significantly differentially expressed genes对照组
Group基因编号
Gene ID差异倍数
Log2 fold
change基因名称
KO_name基因定义
KO_definitionTarCK/TNCK DN74111_c0_g1_i2 −6.76 FOLK 法呢醇醇激酶 Farnesol kinase TarCK/TNCK DN42826_c0_g2_i2 −6.56 PLD1_2 磷脂酶D1/2 Phospholipase D1/2 TarCK/TNCK DN73032_c0_g2_i1 −3.14 ATPeF1B 膜上ATP合酶 F-type H+-transporting ATPase subunit beta TarCK/TNCK DN59908_c0_g1_i2 −1.88 KCS 3-酮脂酰辅酶A合成酶基因 3-ketoacyl-CoA synthase TarCK/TNCK DN88233_c0_g1_i2 −1.08 ENO 烯醇酶 Enolase TarCK/TNCK DN45172_c0_g1_i1 −1.00 CALM 钙调蛋白 Calmodulin TarCK/TNCK DN75575_c0_g1_i1 6.00 AAO3 脱落醛氧化酶 Abscisic-aldehyde oxidase TNCK/TNCa DN75425_c0_g1_i6 −4.83 ABF ABA响应元件结合因子 ABA responsive element binding factor TNCK/TNCa DN89025_c0_g1_i3 −2.34 MFP2 烯酰辅酶A水合酶/3-羟酰辅酶A脱氢酶 Enoyl-CoA hydratase/3-hydroxyacyl-CoA dehydrogenase TNCK/TNCa DN10149_c0_g1_i1 1.09 AUX1 生长素流入载体蛋白 Auxin influx carrier TNCK/TNCa DN36010_c0_g1_i1 1.20 CALM 钙调蛋白 Calmodulin TNCK/TNCa DN81084_c0_g4_i2 1.29 CPK 钙依赖性蛋白激酶 Calcium-dependent protein kinase TNCK/TNCa DN132576_c0_g1_i1 1.64 CML 钙结合蛋白CML Calcium-binding protein CML TNCK/TNCa DN55671_c1_g1_i8 2.01 PLD1_2 磷脂酶D1/2 Phospholipase D1/2 TNCK/TNCa DN1778_c0_g3_i1 2.45 SORD L-艾杜糖醇-2-脱氢酶 L-iditol 2-dehydrogenase TNCK/TNCa DN48191_c0_g1_i1 2.61 EIN2 乙烯不敏感蛋白2 Ethylene-insensitive protein 2 而在叶烧叶片喷钙后即TNCK/TNCa对比分析中,表达量上调的基因有CALM基因、CPK基因(Calcium-dependent protein kinase 钙依赖性蛋白激酶)、EIN2基因 (ethylene-insensitive protein 2 乙烯不敏感蛋白2)[15]、AUX1基因(Auxin influx carrier 生长素流入载体)[16]、PLD1_2基因和果糖和甘露糖的代谢途径的SORD基因(L-iditol 2-dehydrogenase L-艾杜糖醇-2-脱氢酶)[17]。与此同时下调表达的基因有ABF基因(ABA responsive element binding factor ABA响应元件结合因子)[18]和参与脂肪酸降解途径的MFP2基因 (Enoyl-CoA hydratase/3-hydroxyacyl-CoA dehydrogenase烯酰辅酶A水合酶/3-羟酰辅酶A脱氢酶)[19]。
3. 讨论与结论
通过百合叶片扫描电镜分析,叶片近轴面表皮细胞大小随叶烧程度的加深而减小,重度叶烧时,表皮细胞发生皱缩,表明此时表皮细胞失水。叶片远轴面表皮细胞在轻度叶烧时发生皱缩,而近轴面表皮细胞在重度叶烧时才发生皱缩,说明叶烧症状先出现在远轴面一侧。通过透射电镜分析,百合叶片在发生叶烧的过程中,液泡失水是导致表皮细胞体积缩小的原因。因此,推测细胞膜和液泡膜的通透性发生改变是导致百合叶片叶烧病症状的原因。
差异表达基因的PCA分析发现,正常叶片3个重复处理和叶烧叶片3个重复处理之间的差异性都很大,而正常叶片喷钙后3各处理和叶烧叶片喷钙后3个处理之间差异性较小。同时在对比分析中也发现,TarCa/TNCa比较组差异表达基因显著少于TarCK与TNCK,都说明喷钙处理能降低正常叶片和叶烧叶片之间的差异表达基因数量,甚至喷钙处理能够降低正常叶片和叶烧叶片之间差异表达基因数量,说明喷钙处理能缓解叶烧病的发病过程,这与白菜干烧心[20-21]、莴苣[22]等缺钙生理性病害的一致。
在分析单个基因表达中发现,在叶烧病发病过程中,响应钙离子信号CALM基因显著下调,而喷钙处理后CALM和CPK基因显著上调,说明CALM基因是叶烧病发病过程中的主要信号传导基因。同时,叶烧病发病过程中促进生成脱落酸的AAO基因显著上调,叶烧叶片喷钙后乙烯信号调控基因EIN2和生长素输入载体基因AUX1表达量上调,表明叶烧病发病可能受脱落酸、乙烯和生长素的调控。同时,叶烧病发病过程中,在细胞膜和液泡膜稳定相关基因phospholipase D1/2、ATPeF1B 、KCS等表达均显著下调。而喷钙后phospholipase D1/2 显著上调,这与拟南芥中PLD及其产物磷脂酸(PA)调控ABA响应的负调控因子[23-24]结果一致。
钙在植物生理活动中,既起着结构成分的作用,也具有酶的辅助因素功能,它能维持细胞壁、细胞膜及膜结合蛋白的稳定性,参与细胞内各种生长发育的调控作用。因此目前虽然基本证实百合叶烧病是由缺钙导致,但其具体的致病分子机理仍不能十分明晰,今后仍需开展相关基因分析验证工作,为百合分子育种提供理论支撑。
-
表 1 不同AMF对盐胁迫下番茄菌根依赖性及侵染密度的影响
Table 1 Effects of AMF on mycorrhizal dependence and infection density of tomato plants under salt stress
项目
Items处理组
TreatmentsF.m R.i C.e D.v 菌根依赖性
Mycorrhizal
dependence/%0 mmol·L−1 54.79±0.09 a 50.87±0.01 ab 29.07±0.01 c 49.05±0.02 b 100 mmol·L−1 129.06±0.11 a 128.09±0.24 a 111.37±0.15 b 127.08±0.33 ab 侵染密度
Infection
density/%0 mmol·L−1 4.01±0.24 a 4.02±0.11 ab 0.76±0.09 c 3.01±0.07 b 100 mmol·L−1 0.79±0.01 a 0.82±0.03 b 0.13±0.02 c 0.80±0.04 b 注:同项同行数据后不同字母表示差异显著( P <0.05),下同。
Note: Values followed by different lowercase letters within a column indicate significant difference at 5% level, the same as below.表 2 盐胁迫下AMF处理后番茄抗氧化酶系统的变化
Table 2 Changes on antioxidant enzymes of tomato plants under AMF-treatment against salt stress
测定指标
Measured
indicators处理组
Treatments时间 Times/d 15 30 45 SOD/
(U·g−1·min−1 )CK 0 4.76±0.24 e 5.09±0.15 d 4.74±0.19 e R.i 0 5.34±0.08 e 5.49±0.12 cd 5.55±0.08 d F.m 0 6.17±0.26 d 6.30±0.26 c 6.17±0.23 c CK 100 7.29±0.28 c 9.73±0.43 b 7.05±0.10 b R.i 100 8.37±0.16 b 10.58±0.41 b 7.41±0.40 b F.m 100 9.99±0.34 a 12.21±0.30 a 8.46±0.17 a POD/
(U·g−1·min−1 )CK 0 73.61±4.37 d 74.91±3.64 d 85.30±3.67 f R.i 0 83.00±2.33 dc 137.14±3.34 c 132.22±4.69 e F.m 0 84.31±1.63 c 146.53±4.69 c 148.79±3.18 d CK 100 273.11±2.73 b 422.00±3.93 b 565.50±4.13 c R.i 100 347.73±2.71 a 424.44±2.48 b 614.80±3.84 b F.m 100 355.44±3.83 a 468.63±4.00 a 649.68±5.54 a CAT/
(U·g−1·min−1 )CK 0 33.13±1.11 d 35.17±1.06 d 37.13±1.32 e R.i 0 37.86±2.15 cd 37.79±1.69 d 43.54±1.33 d F.m 0 40.32±1.93 c 44.72±1.51 c 49.28±1.23 d CK 100 48.74±1.76 b 74.68±1.79 b 84.25±2.28 c R.i 100 54.25±1.34 b 78.19±1.47 b 93.07±2.80 b F.m 100 61.14±2.63 a 88.19±1.98 a 99.25±1.68 a 表 3 盐胁迫下AMF处理后脯氨酸及MDA含量的变化
Table 3 Proline and MDA contents after AMF-treatment under salt stress
测定指标
Measured
indicators处理组
Treatments时间 Times/d 15 30 45 丙二醛含量
MDA content/
(mmol·g−1)CK 0 3.94±0.14 d 6.81±0.13 d 7.25±0.18 d CK 100 7.28±0.23 a 10.44±0.15 a 17.68±0.38 a F.m 0 3.91±0.15 e 6.76±0.10 d 6.88±0.20 d F.m 100 4.56±0.15 c 8.96±0.19 c 12.37±0.34 c R.i 0 3.93±0.10 de 6.79±0.16 d 7.16±0.16 d R.i 100 6.02±0.17b 9.59±0.21 b 14.12±0.29 b 脯氨酸含量
Pro content/
(μg·g−1)CK 0 42.41±1.58 d 6.81±0.13 cd 7.25±0.18 bc CK 100 35.82±2.06 d 10.44±0.15 d 17.68±0.38 d F.m 0 41.00±3.55 d 6.76±01.0 d 6.88±0.20 c F.m 100 228.57±4.33 a 8.96±0.19 a 12.37±0.34 a R.i 0 118.06±4.18 c 6.79±0.16 c 7.16±0.16 d R.i 100 181.43±3.32 b 9.59±0.21 b 14.12±0.29 b 表 4 不同处理组番茄盐害指数的影响变化
Table 4 Changes on salt injury index of tomato plants under treatments
处理组
Treatments盐害指数
Salt injury indexCK 70.26±2.23 a F.m 35.63±5.15 c R.i 51.23±3.33 b -
[1] 解雪峰, 濮励杰, 沈洪运,等 滨海重度盐碱地改良土壤盐渍化动态特征及预测[J/OL]. 土壤学报: 1-13[2022-02-22]. http://kns.cnki.net/kcms/detail/32.1119.P.20210928.0907.004.html. XIE X F, PU L J, SHEN H Y, et al. Dynamic characteristics and prediction of soil salinization in coastal severely saline-alkali land[J/OL]. Acta Pedologica Sinica: 1-13[2022-02-22]. http://kns.cnki.net/kcms/detail/32.1119.P.20210928.0907.004.html.
[2] ABDEL LATEF A A H, HE C X. Effect of arbuscular mycorrhizal fungi on growth, mineral nutrition, antioxidant enzymes activity and fruit yield of tomato grown under salinity stress [J]. Scientia Horticulturae, 2011, 127(3): 228−233. DOI: 10.1016/j.scienta.2010.09.020
[3] KITAMURA Y, YANO T, HONNA T, et al. Causes of farmland salinization and remedial measures in the Aral Sea Basin—Research on water management to prevent secondary salinization in rice-based cropping system in arid land [J]. Agricultural Water Management, 2006, 85(1/2): 1−14.
[4] ATTIA H, KARRAY N, RABHI M, et al. Salt-imposed restrictions on the uptake of macroelements by roots of Arabidopsis thaliana [J]. Acta Physiologiae Plantarum, 2008, 30(5): 723−727. DOI: 10.1007/s11738-008-0172-4
[5] 郭兆晖. 生态文明建设“十四五”规划与二〇三五远景目标 [J]. 领导科学论坛, 2020(20):3−32. GUO Z H. The 14th five-year plan of ecological civilization construction and the long-term goal of 2035 [J]. The Forum of Leadership Science, 2020(20): 3−32.(in Chinese)
[6] 徐钰德, 刘子金, 程慧, 等. 基于HYDRUS-3D的畦灌模式下田间水盐运移模拟 [J]. 水利水电技术(中英文), 2021(7):14−22. XU Y D, LIU Z J, CHENG H, et al. Simulation of water and salt transportation under border irrigation in field scale based on HYDRUS-3D [J]. Water Resources and Hydropower Engineering, 2021(7): 14−22.(in Chinese)
[7] 潘晶, 黄翠华, 罗君, 等. 盐胁迫对植物的影响及AMF提高植物耐盐性的机制 [J]. 地球科学进展, 2018, 33(4):361−372. DOI: 10.11867/j.issn.1001-8166.2018.04.0361 PAN J, HUANG C H, LUO J, et al. Effects of salt stress on plant and the mechanism of arbuscular mycorrhizal fungi enhancing salt tolerance of plants [J]. Advances in Earth Science, 2018, 33(4): 361−372.(in Chinese) DOI: 10.11867/j.issn.1001-8166.2018.04.0361
[8] ZHANG X H, HAN C Z, GAO H M, et al. Comparative transcriptome analysis of the garden Asparagus (Asparagus officinalis L. ) reveals the molecular mechanism for growth with arbuscular mycorrhizal fungi under salinity stress [J]. Plant Physiology and Biochemistry, 2019, 141: 20−29. DOI: 10.1016/j.plaphy.2019.05.013
[9] 李胜, 姜丽娜, 宋洁蕾, 等. 幼套球囊霉接种量对紫茎泽兰生长的影响 [J]. 云南农业大学学报(自然科学), 2019, 34(2):193−199. LI S, JIANG L N, SONG J L, et al. Effects of arbuscular mycorrhizal fungi, Glomus etunicatum inoculation and different inoculum concentrations on the growth of Ageratina adenophora sprengel [J]. Journal of Yunnan Agricultural University, 2019, 34(2): 193−199.(in Chinese)
[10] 周霞, 崔明, 秦永胜, 等. 扩繁条件对3种丛枝菌根真菌(AMF)的影响 [J]. 中国农学通报, 2012, 28(12):83−87. DOI: 10.11924/j.issn.1000-6850.2011-3888 ZHOU X, CUI M, QIN Y S, et al. The effects of the propagation condition on the three kinds of arbuscular mycorrhizal fungi [J]. Chinese Agricultural Science Bulletin, 2012, 28(12): 83−87.(in Chinese) DOI: 10.11924/j.issn.1000-6850.2011-3888
[11] SMITH S E, JAKOBSEN I, GRØNLUND M, et al. Roles of arbuscular mycorrhizas in plant phosphorus nutrition: Interactions between pathways of phosphorus uptake in arbuscular mycorrhizal roots have important implications for understanding and manipulating plant phosphorus acquisition [J]. Plant Physiology, 2011, 156(3): 1050−1057. DOI: 10.1104/pp.111.174581
[12] 曹荷莉, 丁日升, 薛富岚. 不同水盐胁迫对番茄生长发育和产量的影响研究 [J]. 灌溉排水学报, 2019(2):29−35. CAO H L, DING R S, XUE F L. Growth and yield of tomato as impacted by salinity stress [J]. Journal of Irrigation and Drainage, 2019(2): 29−35.(in Chinese)
[13] 雷垚, 伍松林, 郝志鹏, 等. 丛枝菌根根外菌丝网络形成过程中的时间效应及植物介导作用 [J]. 西北植物学报, 2013, 33(1):154−161. DOI: 10.3969/j.issn.1000-4025.2013.01.024 LEI Y, WU S L, HAO Z P, et al. Development of arbuscular mycorrhizal hyphal networks mediated by different plants and the time effects [J]. Acta Botanica Boreali-Occidentalia Sinica, 2013, 33(1): 154−161.(in Chinese) DOI: 10.3969/j.issn.1000-4025.2013.01.024
[14] 李小方, 张志良. 植物生理学实验指导[M]. 5版. 北京: 高等教育出版社, 2016. [15] SHABNAM N, TRIPATHI I, SHARMILA P, et al. A rapid, ideal, and eco-friendlier protocol for quantifying proline [J]. Protoplasma, 2016, 253(6): 1577−1582. DOI: 10.1007/s00709-015-0910-6
[16] 王仁杰, 朱凡, 梁惠子, 等. 重金属Mn对苦楝叶片光系统性能的影响 [J]. 生态学报, 2020, 40(6):2019−2027. WANG R J, ZHU F, LIANG H Z, et al. Effects of Manganese (Mn) on the performances of photosystems Ⅰ and Ⅱ in Melia azedarach young plant [J]. Acta Ecologica Sinica, 2020, 40(6): 2019−2027.(in Chinese)
[17] TORRES R, DIZ V E, LAGORIO M G. Effects of gold nanoparticles on the photophysical and photosynthetic parameters of leaves and chloroplasts [J]. Photochemical & Photobiological Sciences, 2018, 17(4): 505−516.
[18] 李翔, 桑勤勤, 束胜, 等. 外源油菜素内酯对弱光下番茄幼苗光合碳同化关键酶及其基因的影响 [J]. 园艺学报, 2016, 43(10):2012−2020. LI X, SANG Q Q, SHU S, et al. Effects of epibrassinolide on the activities and gene expression of photosynthetic enzymes in tomato seedlings under low light [J]. Acta Horticulturae Sinica, 2016, 43(10): 2012−2020.(in Chinese)
[19] 杨凤军, 高凤, 韩昱, 等. 不同基因型番茄幼苗期耐盐性分析 [J]. 黑龙江八一农垦大学学报, 2018, 30(4):12−17. DOI: 10.3969/j.issn.1002-2090.2018.04.003 YANG F J, GAO F, HAN Y, et al. Analysis of salt tolerance of different genotypic tomato seedlings [J]. Journal of Heilongjiang August First Land Reclamation University, 2018, 30(4): 12−17.(in Chinese) DOI: 10.3969/j.issn.1002-2090.2018.04.003
[20] NEMEC S. Response of six citrus root-stocks to three species of Glomus, a mycorrhizalfungus [J]. Proc Fla State Hort Sci, 1978, 9(1): 10−14.
[21] 罗巧玉, 王晓娟, 林双双, 等. AM真菌对重金属污染土壤生物修复的应用与机理 [J]. 生态学报, 2013, 33(13):3898−3906. LUO Q Y, WANG X J, LIN S S, et al. Mechanism and application of bioremediation to heavy metal polluted soil using arbuscular mycorrhizal fungi [J]. Chinese Journal of Plant Ecology, 2013, 33(13): 3898−3906.(in Chinese)
[22] 何新华, 段英华, 陈应龙, 等. 中国菌根研究60年: 过去、现在和将来 [J]. 中国科学(生命科学), 2012, 42(6):431−454. HE X H, DUAN Y H, CHEN Y L, et al. A 60-year journey of mycorrhizal research in China: Past, present and future directions [J]. Science in China (Series C), 2012, 42(6): 431−454.(in Chinese)
[23] WU Q S, ZOU Y N, HE X H. Contributions of arbuscular mycorrhizal fungi to growth, photosynthesis, root morphology and ionic balance of Citrus seedlings under salt stress [J]. Acta Physiologiae Plantarum, 2010, 32(2): 297−304. DOI: 10.1007/s11738-009-0407-z
[24] 曹翠玲, 张玖玲, 杨向娜, 等. 金银花根系VAM真菌侵染过程观察 [J]. 西北植物学报, 2016, 36(3):479−485. DOI: 10.7606/j.issn.1000-4025.2016.03.0479 CAO C L, ZHANG J L, YANG X N, et al. Investigation of the mycorrhiza forming in honeysuckle infected by vesicular arbuscular mycorrhizal(VAM) [J]. Acta Botanica Boreali-Occidentalia Sinica, 2016, 36(3): 479−485.(in Chinese) DOI: 10.7606/j.issn.1000-4025.2016.03.0479
[25] LOTFI R, PESSARAKLI M, GHARAVI-KOUCHEBAGH P, et al. Physiological responses of Brassica napus to fulvic acid under water stress: Chlorophyll a fluorescence and antioxidant enzyme activity [J]. The Crop Journal, 2015, 3(5): 434−439. DOI: 10.1016/j.cj.2015.05.006
[26] HE Z Q, HE C X, ZHANG Z B, et al. Changes of antioxidative enzymes and cell membrane osmosis in tomato colonized by arbuscular mycorrhizae under NaCl stress [J]. Colloids and Surfaces B:Biointerfaces, 2007, 59(2): 128−133. DOI: 10.1016/j.colsurfb.2007.04.023
[27] GUO S X, CHEN D M, RUNJIN L. Effects of arbuscular mycorrhizal fungi on antioxidant enzyme activity in peony seedlings under salt stress [J]. Acta Horticulturae Sinica, 2010, 37(11): 1796−1802.
[28] KAUR G, ASTHIR B. Proline: a key player in plant abiotic stress tolerance [J]. Biologia Plantarum, 2015, 59(4): 609−619. DOI: 10.1007/s10535-015-0549-3
[29] CAO D, CHEN S, HUANG Y, et al. Effects of artificial aging on physiological characteristics of rice seeds with different dormancy characteristics [J]. Agricultural Biotechnology, 2019, 8(1): 56−60.
[30] CHANDRASEKARAN M, BOUGHATTAS S, HU S J, et al. A meta-analysis of arbuscular mycorrhizal effects on plants grown under salt stress [J]. Mycorrhiza, 2014, 24(8): 611−625. DOI: 10.1007/s00572-014-0582-7
[31] 冯固, 白灯莎, 杨茂秋, 等. 盐胁迫对VA菌根形成及接种VAM真菌对植物耐盐性的效应 [J]. 应用生态学报, 1999, 10(1):79−82. DOI: 10.3321/j.issn:1001-9332.1999.01.021 FENG G, BAI D S, YANG M Q, et al. Effects of salt stress on VA mycorrhizal formation and inoculation of VAM fungi on plant salt tolerance [J]. Chinese Journal of Applied Ecology, 1999, 10(1): 79−82.(in Chinese) DOI: 10.3321/j.issn:1001-9332.1999.01.021
[32] 邹晖, 林江波, 戴艺民, 等. 干旱胁迫下内生真菌对铁皮石斛抗旱性的影响 [J]. 北方园艺, 2020(6):119−125. ZOU H, LIN J B, DAI Y M, et al. Effects of endophyte on the drought resistance of Dendrobium officinale under drought stress [J]. Northern Horticulture, 2020(6): 119−125.(in Chinese)
[33] 吴秀红, 戚厚芸, 孙婷, 等. 内生菌根菌剂对水稻秧苗生长及生理特性的影响 [J]. 江苏农业科学, 2018, 46(21):65−68. WU X H, QI H Y, SUN T, et al. Effects of endogenous mycorrhizal fungi inoculant on growth and physiological characteristics of rice seedlings [J]. Jiangsu Agricultural Sciences, 2018, 46(21): 65−68.(in Chinese)
[34] MA H, WANG A, ZHANG M H, et al. Compared the physiological response of two petroleum-tolerant contrasting plants to petroleum stress [J]. International Journal of Phytoremediation, 2018, 20(10): 1043−1048. DOI: 10.1080/15226514.2018.1460303
[35] TEH C Y, SHAHARUDDIN N A, HO C L, et al. Exogenous proline significantly affects the plant growth and nitrogen assimilation enzymes activities in rice (Oryza sativa) under salt stress [J]. Acta Physiologiae Plantarum, 2016, 38(6): 1−10.
[36] ELHINDI K M, EL-DIN A S, ELGORBAN A M. The impact of arbuscular mycorrhizal fungi in mitigating salt-induced adverse effects in sweet basil (Ocimum basilicum L. ) [J]. Saudi Journal of Biological Sciences, 2017, 24(1): 170−179. DOI: 10.1016/j.sjbs.2016.02.010
[37] 张斌斌, 许建兰, 蔡志翔, 等. 淹水胁迫下2个李砧木品种光合特性变化及其与环境因子的关系 [J]. 南京农业大学学报, 2013(5):39−44. ZHANG B B, XU J L, CAI Z X, et al. Relationship between photosynthetic characteristics and environmental factors in leaves of two plum rootstock varieties under waterlogging stress [J]. Journal of Nanjing Agricultural University, 2013(5): 39−44.(in Chinese)
[38] LYSENKO V S, VARDUNY T V, KOSENKO P O, et al. Video registration as a method for studying kinetic parameters of chlorophyll fluorescence in Ficus benjamina leaves [J]. Russian Journal of Plant Physiology, 2014, 61(3): 419−425. DOI: 10.1134/S102144371403008X
[39] MAURO R P, OCCHIPINTI A, LONGO A M G, et al. Effects of shading on chlorophyll content, chlorophyll fluorescence and photosynthesis of subterranean clover [J]. Journal of Agronomy and Crop Science, 2011, 197(1): 57−66. DOI: 10.1111/j.1439-037X.2010.00436.x
[40] 朱先灿, 宋凤斌, 徐洪文. 低温胁迫下丛枝菌根真菌对玉米光合特性的影响 [J]. 应用生态学报, 2010, 21(2):470−475. ZHU X C, SONG F B, XU H W. Effects of arbuscular mycorrhizal fungi on photosynthetic characteristics of maize under low temperature stress [J]. Chinese Journal of Applied Ecology, 2010, 21(2): 470−475.(in Chinese)
[41] 刘建新, 欧晓彬, 王金成. 镧胁迫下外源H2O2对裸燕麦幼苗叶绿素荧光参数和光合碳同化酶活性的影响 [J]. 生态学报, 2019, 39(8):2833−2841. LIU J X, OU X B, WANG J C. Effects of exogenous hydrogen peroxide on chlorophyll fluorescence parameters and photosynthetic carbon assimilation enzymes activities in naked oat seedlings under lanthanum stress [J]. Acta Ecologica Sinica, 2019, 39(8): 2833−2841.(in Chinese)
[42] 刘领, 李冬, 马宜林, 等. 外源褪黑素对干旱胁迫下烤烟幼苗生长的缓解效应与生理机制研究 [J]. 草业学报, 2019, 28(8):95−105. DOI: 10.11686/cyxb2019098 LIU L, LI D, MA Y L, et al. Alleviation of drought stress and the physiological mechanisms in tobacco seedlings treated with exogenous melatonin [J]. Acta Prataculturae Sinica, 2019, 28(8): 95−105.(in Chinese) DOI: 10.11686/cyxb2019098
[43] 张淑彬, 纪晶晶, 王幼珊, 等. 内蒙古露天煤矿区回填土壤具生态适应能力丛枝菌根真菌的筛选 [J]. 生态学报, 2009, 29(7):3729−3736. DOI: 10.3321/j.issn:1000-0933.2009.07.034 ZHANG S B, JI J J, WANG Y S, et al. The screening of arbuscular mycorrhizal fungi with high ecological adaptations in backfill soil of open pit mining area in Inner Mongolia [J]. Acta Ecologica Sinica, 2009, 29(7): 3729−3736.(in Chinese) DOI: 10.3321/j.issn:1000-0933.2009.07.034





 下载:
下载: