Determination of Viability of Passion Fruit Pollens
-
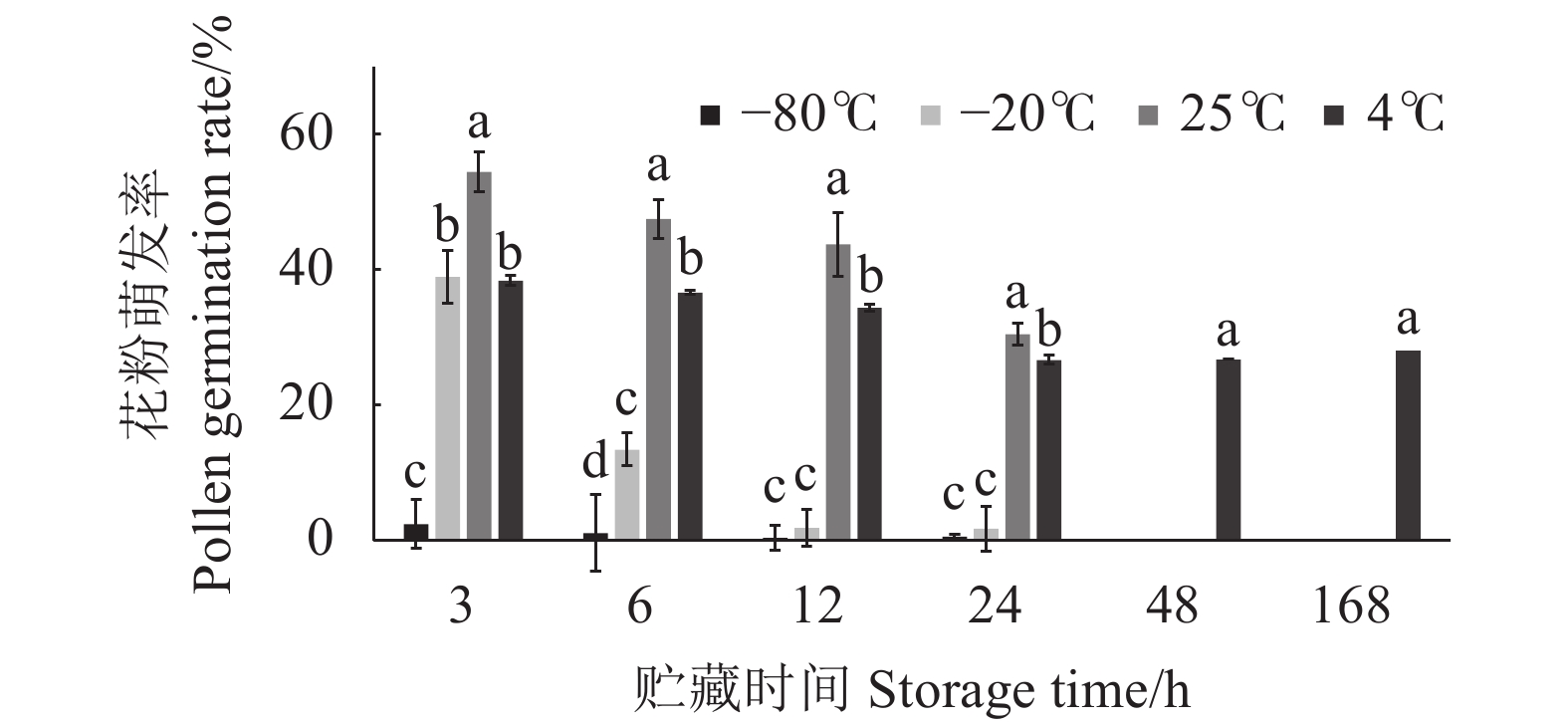
摘要:目的 明确西番莲属植物花粉最佳离体培养基、离体培养最适观测时间、花粉的最适染色法和花粉贮藏条件,快速精准鉴定西番莲属种质资源的花粉活力,为其种质资源利用与新品种选育提供科学依据。方法 以6份西番莲属种质资源的花粉为试验材料,设计正交试验,探究不同浓度的蔗糖、H3BO3、Ca(NO3)2和聚乙二醇-4000(PEG-4000)对花粉离体萌发率的影响;利用最佳花粉离体培养基,筛选花粉离体培养最适观测时间;采用I2-KI染色法、TTC染色法与亚历山大染色法,探究最适的西番莲花粉染色法;通过不同贮藏温度与时间处理,筛选花粉的最佳贮藏条件。结果 正交试验筛选出最佳培养基配方为100 g·L−1 蔗糖+0.04 g·L−1 H3BO3+0.01 g·L−1 Ca(NO3)2+150 g·L−1 PEG-4000+200 mg·L−1 MgSO4·7H2O+100 mg·L−1 KNO3,pH值5.5;花粉离体培养1 h为最适观测时间;TTC染色法染色效果好且结果与离体萌发率无显著差异,可有效检测西番莲花粉活力;花粉贮藏24 h以内,25 ℃与4 ℃的贮藏温度下花粉活力分别为30.48%、26.69%,花粉贮藏时间在1~7 d,4 ℃下花粉活力仍保持在26.54%~27.05%。结论 筛选出西番莲属植物花粉离体萌发的最佳培养基组合、离体培养最适观测时间、花粉最适染色法与花粉贮藏最佳条件,为西番莲属植物花粉活力的快速筛选和品种改良提供理论依据。Abstract:Objective Method for determining the viability of passion fruit pollens was explored to facilitate the utilization of existing germplasms and breeding new varieties.Methods Medium and conditions for in vitro culture of Passiflora pollens was optimized. Appropriate staining methods to determine and storage conditions to maximize the pollen viability were investigated. Contents of sucrose, H3BO3, Ca(NO3)2, and polyethylene glycol-4000 (PEG-4000) of the medium were optimized in an orthogonal experiment on pollens of 6 germplasms. Pollen germination on the finalized medium for varied culture times was observed. The staining of I2-KI, TTC, and Alexander methods and storage for different durations at different temperatures were compared.Results The optimum medium was formulated with 100 g·L−1 sucrose, 0.02 g·L−1 H3BO3, 0.04 g·L−1 Ca(NO3)2, 150 g·L−1 PEG-4000, 200 g·L−1 MgSO4·7H2O, and 100 g·L−1 KNO3, then adjusted to pH 5.5. The pollen germination was examined in 1 h of the in vitro culture. TTC staining showed satisfactory effect with no significant deviations from the in vitro observation indicating it an applicable indicator for pollen viability. In 24 h of storage at 25 ℃ the pollen viability was 30.48%; and at 4 ℃, it was 26.69% and remained 26.54%–29.05% for 7 d.Conclusion The medium formulation and in vitro culture time as well as the staining method and storage conditions of passion fruit pollens were determined making a rapid and reliable procedure to maximize the pollen germination available.
-
Keywords:
- Passion fruit /
- pollen viability /
- culture medium /
- in vitro pollen germination /
- staining /
- pollens storage
-
0. 引言
【研究意义】二氧化硫是葡萄酒等果酒酿造中常用的添加剂,具有防止酒体过度氧化、挥发酸含量上升和抑制杂菌生长等作用[1]。但高量的二氧化硫(60 mg·L−1以上)会对酒体中的乳酸菌产生胁迫作用,使其生长受抑制甚至死亡,影响了果酒的苹果酸-乳酸发酵(Malolactic fementation,MLF)生物降酸效果和酒体品质提升[2];而低量的二氧化硫又不能充分发挥作用,从而易导致酒体品质劣变;因此高耐受二氧化硫的MLF乳酸菌的选择备受关注。植物乳杆菌(Lactobacillus plantarum)R23是本试验人员从自然发酵的枇杷酒中分离出的高耐硫、高抗逆性优良MLF乳酸菌,已在山葡萄、枇杷和杨梅等高酸性水果的酿造中广泛应用,成为果酒生物降酸中的重要菌株[3-4]。【前人研究进展】已有研究表明,二氧化硫在液体介质中以HSO3−、SO32−等形式存在,这些离子转化为SO42−的过程会产生活性氧簇(Reactive oxygen species,ROS)如∙O2ˉ、H2O2、∙OH等;持续增加的ROS将作用于细胞内生物大分子进而诱发核酸、蛋白质和脂质等的氧化损伤[5-6]。但同时,生物体会启动氧化防御系统以清除过多的ROS进而维持其正常的生长代谢,即所谓的氧化应激。事实上,强氧化环境均会导致生物体内ROS大量积累,引发生物体氧化应激反应[7],如槲皮素、芦丁等诱发了嗜酸乳杆菌NCFM氧化应激,且主要依靠过氧化氢酶的作用清除胞内自由基[8];另外,在李斯特氏菌的氧化应激反应中,超氧化物歧化酶活性和DNA损伤修复主导了自由基的清除过程[9]。【本研究切入点】推测二氧化硫胁迫可能引发植物乳杆菌R23氧化应激反应,且其抗氧化相关系统发挥了积极作用,但迄今植物乳杆菌R23耐受二氧化硫的应激机制尚不明确。【拟解决的关键问题】本研究从胞内抗氧化酶活力、细胞形态、细胞膜丙二醛和磷脂脂肪酸含量等方面进行探究,明确二氧化硫胁迫下植物乳杆菌R23生理代谢变化,以期揭示植物乳杆菌R23对二氧化硫胁迫的适应性机制。
1. 材料与方法
1.1 试验材料
1.1.1 菌株
植物乳杆菌(Lactobacillus plantarum)R23,由本实验室人员从自然发酵的枇杷酒中分离并保存,NCBI号为HQ58056。
1.1.2 培养基
LHR20液体培养基:酵母膏0.74%,牛肉膏1.0%,蛋白胨0.5%,蔗糖2.0%,柠檬酸铵0.2%,MgSO4 0.036%,MnSO4 0.005%,吐温-80 0.1%,苹果酸钠5.7%(体积分数35%),番茄汁5%,平菇浸汁5%,0.2 mol·L−1 Na2HPO4-0.2 mol·L−1 KH2PO4 缓冲盐稀释4倍,121 ℃湿热灭菌20 min。
1.1.3 主要仪器
SCIENTZ-950E细胞破碎仪(宁波新芝生物科技股份有限公司),SPX-250BS-Ⅱ型生化培养箱(上海新苗医疗器械制造有限公司),SW-CJ-IFD型单人单面净化工作台(苏州净化设备有限公司),TGL-18C型高速冷冻离心机(上海安亭科学仪器厂),GI54DW型高压灭菌器(美国致微),6380LV型扫描电镜(日本电子公司),BioTek Epoch2全波长酶标仪(Bio Tek), UV-1705紫外可见分光光度计(日本岛津),Agilent7890N型气相色谱系统(美国MIDI公司)。
1.1.4 主要试剂
(1)抗氧化酶活力测定及丙二醛含量测定试剂盒(上海起子生物科技有限公司)。(2)磷脂脂肪酸提取试剂。皂化试剂(试剂1):NaOH 45 g + 甲醇(HPLC grade)150 mL+去离子蒸馏水150 mL和甲醇混合后加入NaOH中,同时搅拌至完全溶解;甲基化试剂(试剂2):6.0 mol·L−1盐酸 325 mL+甲醇(HPLC grade)275 mL把盐酸加入到甲醇中,并不断搅拌;萃取试剂(试剂3):正己烷 (HPLC Grade ) 200 mL+MTBE (HPLC Grade ) 200 mL,把MTBE加入到正己烷中,并搅拌均匀;洗涤试剂(试剂4):NaOH 10.8 g+去离子蒸馏水900 mL。
1.2 试验方法
1.2.1 菌种活化
将植物乳杆菌R23甘油冻存种以5%(V/V)接种于LHR20中,30 ℃培养15~18 h,连续转接活化2次,备用。
1.2.2 菌体二氧化硫胁迫培养
将植物乳杆菌R23活化种以体积分数为5%接种于二氧化硫质量浓度分别为0、40、80、120 mg·L−1的 LHR20液体培养基中,30 ℃恒温静置培养4 h后,备用。
1.2.3 菌体处理与形态观察
取1 mL不同质量浓度二氧化硫胁迫处理后的菌液于1.5 mL无菌离心管中,5000 r·min−1离心3 min,用无菌水洗涤2次后,用适量2.5%戊二醛(pH 7.0)固定,后续制样、扫描电镜观察均在福建省农业科学院农业质量标准与检测技术研究所进行。
1.2.4 抗氧化酶活力测定样品制备
取50 mL不同质量浓度二氧化硫胁迫处理的植物乳杆菌R23菌悬液,以9000 r·min−1、4 ℃离心10 min,弃上清;加无菌生理盐水洗涤1次;菌泥中加入5 mL PBS(pH8.0)缓冲液,加玻璃珠旋涡震荡混匀;然后进行超声破碎,条件为:功率400 W、工作总时间5 min、工作1 s、间歇2 s、变幅杆直径5 mm;细胞破碎液经12000 r·min−1、4 ℃离心10 min;将上清液装于10 mL离心管,4 ℃暂存,备用。
1.2.5 蛋白质含量测定
采用考马斯亮蓝法。标准曲线的制作:取6支具塞试管,编号,按表1加入试剂,摇匀,反应10 min,于595 nm处测定吸光度。
表 1 蛋白质含量测定标准曲线Table 1. Standard curve for protein determination试剂
Reagent管号
Tube No.1 2 3 4 5 6 蛋白质标准液
Standard solution of protein/mL0 0.2 0.4 0.6 0.8 1.0 蒸馏水
Distilled water/mL1.0 0.8 0.6 0.4 0.2 0 考马斯亮蓝G-250试剂
Coomassie brilliant blue G-250 reagent/mL5 5 5 5 5 5 样品测定:取样品溶液0.1 mL于试管中,加水0.9 mL,各加入5 mL考马斯亮蓝试剂,充分混合,放置10 min,以试剂空白为对照,595 nm处测定吸光值,同时做3个重复。
1.2.6 酶活力及丙二醛含量测定
超氧化物歧化酶SOD、谷胱甘肽过氧化物酶GPX、过氧化氢酶CAT活力及丙二醛MDA含量的测定,采用试剂盒法,具体操作按照说明书进行。
1.2.7 细胞膜脂肪酸提取
(1)获菌:取植物乳杆菌R23胁迫前、后(分别用0、80 mg·L−1二氧化硫处理)的菌液20 mL,4 ℃、5000 r·min−1,离心5 min,用接种环挑取约40 mg湿重的菌落置于清洁干燥的有螺旋盖的试管(13 mm × 100 mm)底部;(2)皂化:在装有菌体的试管内加入(1.0±0.1)mL试剂1,锁紧盖子,震荡试管5~10 s,95~100 ℃水浴5 min,从沸水中移开试管并轻微冷却,震荡5~10 s,再水浴25 min,取出室温冷却;(3)甲基化:加入(2.0±0.1)mL试剂2,拧紧盖子,震荡5~10 s,80 ℃水浴10 min,移开且快速用流动自来水冷却至室温;(4)萃取:加入(1.25±0.1)mL 试剂3萃取溶剂,盖紧盖子,温和混合旋转10 min ,打开管盖,利用干净的移液管取出下层似水部分,弃去;(5)基本洗涤:加入(3.0±0.21)mL试剂4,拧紧盖子,温和混合旋转5 min,打开盖子,利用干净的移液管移出约2/3体积的上层有机相到干净的GC检体小瓶。
1.2.8 脂肪酸检测
应用MIDI系统,色谱分析柱温采用二阶顺序升温法,即第一阶段170 ℃起始,每分钟升温5 ℃,升至260 ℃,第二阶段每分钟升温40 ℃,升至310 ℃,维持90 s;汽化室温度250 ℃、检测器温度300 ℃;载气为氢气(2 mL·min−1)、尾吹气为氮气(30 mL∙min−1);柱前压68.95 kPa;进样量1 μL,进样分流比100∶1。将检测出的脂肪酸数据转换成以乳酸菌为样本、以脂肪酸生物标记为指标的数据矩阵Excel文件,然后利用生物统计软件SPSS 23.0中的Graphs进行分析。
2. 结果与分析
2.1 二氧化硫胁迫激发的菌体抗氧化防御反应
植物乳杆菌R23在梯度二氧化硫胁迫处理条件下的胞内抗氧化酶活力、膜丙二醛含量和超微形态变化如图1、图2和图3所示。以正常培养条件为对照,二氧化硫胁迫处理后3种抗氧化酶活力均显著提升(P < 0.05),其中80 mg·L−1 二氧化硫胁迫下SOD、CAT、GPX分别是无胁迫处理的1.64、2.14、1.62倍,特别是CAT活力提升速率最快且后续下降幅度最小,提示适量二氧化硫胁迫可有效激发植物乳杆菌R23的抗氧化系统,而CAT可能起了主导作用。当胁迫二氧化硫质量浓度增大到120 mg·L−1时,酶活力略有下降但与最高值无显著差异,且显著高于对照值(P<0.05)(图1)。
![]() 图 1 梯度二氧化硫胁迫下植物乳杆菌R23胞内抗氧化酶活力小写字母表示梯度二氧化硫质量浓度胁迫后菌体抗氧化酶活性差异显著(P<0.05)。Figure 1. Intracellular antioxidase activity of L. plantarum R23 under gradient of SO2 stressData with different lowercase letters represent significant differences on antioxidase activity of L. plantarum R23 treated by gradient of SO2 at P<0.05
图 1 梯度二氧化硫胁迫下植物乳杆菌R23胞内抗氧化酶活力小写字母表示梯度二氧化硫质量浓度胁迫后菌体抗氧化酶活性差异显著(P<0.05)。Figure 1. Intracellular antioxidase activity of L. plantarum R23 under gradient of SO2 stressData with different lowercase letters represent significant differences on antioxidase activity of L. plantarum R23 treated by gradient of SO2 at P<0.05图2显示,梯度二氧化硫胁迫导致MDA含量不断增加;其中40或80 mg·L−1二氧化硫胁迫下MDA变化幅度较小,之后加速上升 (P <0.05),在120 mg·L−1 二氧化硫胁迫下MDA含量是对照处理的1.84倍;说明抗氧化酶活力下降后胞内ROS不能得到有效清除,进而导致菌体脂质过氧化反应加剧。
植物乳杆菌R23在不同质量浓度二氧化硫胁迫下的菌体细胞超微形态如图3所示。正常培养条件下(0 mg·L−1二氧化硫),菌体饱满,呈杆状、单个或短链状排列,不生孢(图3-a);40 mg·L−1二氧化硫胁迫并未导致菌体形态发生肉眼看见的变化(图3-b);但胁迫程度进一步加深后,有些菌体表面发生皱缩现象,细胞失去原有的饱满程度(图3-c、d),其中120 mg·L−1二氧化硫胁迫下这一变化更加明显。可见80 mg·L−1 SO2胁迫下菌体尚可维持基本的形态,而120 mg·L−1二氧化硫胁迫虽未导致抗氧化酶活力显著下降,但其防御性能下降,这不仅表现在MDA含量显著提升,也表现在菌体形态的变化上。
2.2 二氧化硫胁迫诱导的细胞膜磷脂脂肪酸变化
上述研究结果表明,80 mg·L−1二氧化硫胁迫可有效激发植物乳杆菌R23生理应答,且未导致明显的结构损伤,因此考察了该浓度胁迫下菌体细胞膜磷脂脂肪酸的结构变化,结果如表2和图4所示。无胁迫时菌体细胞膜脂肪酸的组成成分包括饱和脂肪酸即十四烷酸(C14:0)、十六烷酸(C16:0)、十八烷酸(C18:0)、十二碳异脂肪酸(iso-C12:0)、十七碳前异脂肪酸(anteiso-C17:0),单不饱和脂肪酸即十六碳异脂肪酸(iso-C16:1)、十六碳顺式脂肪酸(iso-C16:1)和十九碳反式脂肪酸(trans-C19:1),以及环丙烷脂肪酸(cyclo-19:0)。其中C14:0、C16:0、C18:0和cyclo-19:0含量之和超过57.2%,占总脂肪酸的比例较大,特别是C16:0占总量的27%以上,应为植物乳杆菌R23脂肪酸主要组成成分。胁迫条件下,菌体脂肪酸发生不同程度变化,其中饱和脂肪酸即十四碳异脂肪酸(iso-C14:0)、十五碳异脂肪酸(iso-C15:0)和十八碳顺式单不饱和脂肪酸(cis-C18:1)从无到有,而iso-C16:1、anteiso-C17:0从有到无。说明二氧化硫胁迫不仅导致菌体脂肪酸含量发生变化,也调整了脂肪酸的组成成分。
表 2 不同二氧化硫胁迫下植物乳杆菌R23细胞膜磷脂脂肪酸相对含量Table 2. Relative contents of PLFA in L. plantarum R23 under SO2 stress磷脂脂肪酸
PLFA磷脂脂肪酸相对含量
Relative amount of PLFA/%胁迫前
Before stress胁迫后
After stress十二碳异脂肪酸 12:0 iso 1.36±0.08 2.45±0.11 十四烷酸 14:0 7.34±0.21 3.36±0.31 十四碳异脂肪酸 14:0 iso 0 1.92±0.18 十五碳异脂肪酸 15:0 iso 0 0.98±0.04 十六碳异脂肪酸 16:1 iso 3.99±0.18 0 十六碳顺式单不饱和脂肪酸 16:1 cis 3.19±0.55 2.87±0.65 十六烷酸 16:0 27.77±1.29 30.10±1.20 十七碳前异脂肪酸 17:0 anteiso 1.55±0.10 0 Sum In Feature 8 7.35±0.23 12.05±0.92 Sum In Feature 9 18.67±0.11 8.14±0.96 十八烷酸 18:0 8.65±0.59 10.92±0.17 十八碳顺式单不饱和脂肪酸 18:1 cis 0 2.46±0.20 十九碳反式脂肪酸 19:1 trans 2.41±0.16 2.29±0.40 Sum In Feature 11 4.28±0.03 7.80±0.08 环丙烷脂肪酸 19:0 cyclo 13.44±0.31 14.66±0.34 ![]() 图 4 二氧化硫对植物乳杆菌R23细胞膜磷脂脂肪酸相对含量的影响a、b、c、d分别表示二氧化硫胁迫导致的总饱和脂肪酸与总不饱和脂肪酸、总长链脂肪酸与总短链脂肪酸、总直链脂肪酸与总支链脂肪酸、总顺式脂肪酸与总反式脂肪酸的变化;“*”表示两者间的差异显著(P < 0.05)。Figure 4. Effect of SO2 on relative contents of PLFA in L. plantarum R23a, b, c, and d: changes caused by SO2 stress on saturated (Sa-), unsaturated (Us-), long-chain (L-), short-chain (S-), straight-chain (Sc-), branched-chain (Bc-), cis (Cis-), and trans fatty acids (Trans-) PLFAs. “*” indicates significant difference at P<0.05.
图 4 二氧化硫对植物乳杆菌R23细胞膜磷脂脂肪酸相对含量的影响a、b、c、d分别表示二氧化硫胁迫导致的总饱和脂肪酸与总不饱和脂肪酸、总长链脂肪酸与总短链脂肪酸、总直链脂肪酸与总支链脂肪酸、总顺式脂肪酸与总反式脂肪酸的变化;“*”表示两者间的差异显著(P < 0.05)。Figure 4. Effect of SO2 on relative contents of PLFA in L. plantarum R23a, b, c, and d: changes caused by SO2 stress on saturated (Sa-), unsaturated (Us-), long-chain (L-), short-chain (S-), straight-chain (Sc-), branched-chain (Bc-), cis (Cis-), and trans fatty acids (Trans-) PLFAs. “*” indicates significant difference at P<0.05.对不同类型脂肪酸的统计分析发现,二氧化硫胁迫后菌体总饱和脂肪酸含量上升,总不饱和脂肪酸含量下降(图4-a),两者比值提高1.75,即二氧化硫胁迫导致菌体膜脂肪酸饱和程度增加。类似的,二氧化硫胁迫导致了总直链脂肪酸提升(从49.36%升至52.0%),而总支链脂肪酸显著下降(从6.90%降至5.35%,P<0.05),总体直链/支链比值显著提升(从7.15升至9.72,P<0.05);值得注意的是,支链脂肪酸中的异脂肪酸虽未发生变化,但前异脂肪酸从1.55%降低为0,因此,总体异脂肪酸和前异脂肪酸的比值增大(图4-c)。另外,顺式/反式脂肪酸的比值也从1.33显著提升到2.33(P<0.05)(图4-d)。不同的是,菌体长链脂肪酸和短链脂肪酸在二氧化硫胁迫后的相对含量均有微量提升,但长链脂肪酸的增加幅度更大,因此总体上长链/短链的比值提升0.12(图4-b)。另外还可以发现,作为植物乳杆菌R23脂肪酸主要组成成分之一的环丙烷脂肪酸cyclo-C19:0,在胁迫处理后其相对含量也得以提升,提示其在该菌体耐受二氧化硫胁迫过程中发挥了一定的作用。从整体变化趋势分析,二氧化硫胁迫后植物乳杆菌R23膜脂肪酸成分向着饱和、长链、直链和环型调整。
3. 讨论与结论
3.1 抗氧化酶应激酶系对二氧化硫胁迫的响应
微生物体会启动自身应激机制以抵御各种逆境胁迫等不利条件,如产生特殊保护功能的蛋白[10]、改变细胞膜流动性[11-12]等,从而维持菌体正常的生长代谢和功能;本研究则发现植物乳杆菌R23通过提高抗氧化酶活力、调节细胞膜脂肪酸结构的方式来应对二氧化硫胁迫。已有研究发现,在生物体氧化应激反应中SOD、GPX和CAT是清除ROS的重要抗氧化酶类 [13],如将sod基因进行外源表达后,可明显提高乳杆菌的抗氧化能力[14];而发酵乳杆菌ME-3中的谷胱甘肽过化物酶和谷胱甘肽还原酶,可以从环境中运输及合成谷胱甘肽来参与抗氧化反应[15]。本研究植物乳杆菌R23抗氧化酶活力的显著提升表明该菌也启动了氧化应激反应,且其抗氧化系统整体呈现一个主动防御到被破坏的趋势。起始阶段,随着胁迫程度增大,SOD、GPX和CAT活性明显提高(P<0.05),以清除胞内过量的∙O2ˉ、H2O2、∙OH;但当二氧化硫质量浓度超过菌体耐受阈值时,各酶活力开始下降,MDA含量上升幅度加大。这揭示了过高二氧化硫胁迫会导致菌体抗氧化系统失调,使得胞内自由基产生和清除失去动态平衡,过量自由基与细胞膜中的脂肪酸等物质发生脂质过氧化反应,进而引起细胞膜流动性或通透性改变,促使胞内离子和其他电解质及可溶性物质大量外渗,破坏了细胞内酶及代谢作用原有的区域性,最终导致细胞皱缩甚至破裂。因此,抗氧化酶活性对菌体细胞应对氧化损伤起到至关重要的作用。
3.2 细胞膜对二氧化硫胁迫的响应
细胞膜是把菌体细胞与外界环境隔离的第一道屏障,也是逆境因子生理胁迫的首要目标之一,因此在维持胞内微环境的稳定性等方面发挥极其重要的作用[16]。一般认为,细胞膜的生化特性很大程度上取决于组成磷脂的脂肪酸结构[17]。本研究借助MIDI系统对二氧化硫胁迫前后植物乳杆菌R23膜脂肪酸测定结果发现,胁迫后菌体脂肪酸成分及比例均发生变化,整体向着饱和、长链、直链和环型增长,其中直链/支链、顺式/反式的比值显著提升(P<0.05)。由于总直链脂肪酸占比较高(50%以上),因此推断直链(尤其是C16:0)与支链的比例在植物乳杆菌R23防御二氧化硫胁迫中起主导作用。虽然本研究中菌体总饱和脂肪酸和长链脂肪酸含量增加不显著,但其占比也较大,所以认为它们同样发挥了积极作用。另有研究认为,不同类型脂肪酸的比例均会对细胞膜的流动性产生影响[18],其中支链脂肪酸(尤其是前异脂肪酸)中的甲基会破坏酰基链的紧凑性[19],降低膜流动性;如单增李斯特菌依靠增加直链脂肪酸同时降低前异脂肪酸C15:0、C17:0来适应酸性环境[20],而本研究中总支链脂肪酸增加以及前异脂肪酸anteiso-C17:0显著降低(P<0.05)应是植物乳杆菌R23应对二氧化硫胁迫的又一适应性调节方式。另外,长链饱和脂肪酸可以增加磷脂双分子层间酰基链的相互作用力,使其结构更为牢固且具有较高的相变温度和较低的渗透性[17];因此植物乳杆菌R23对长链/短链、饱和/不饱和脂肪酸的调整,可以降低细胞膜对HSO3−、SO32−等的透过性,从而防止ROS大量产生。另外,由于不饱和脂肪酸的氢键易被ROS氧化,本研究中其含量降低可以减弱膜脂对过氧化作用的敏感性。然而,目前关于不同类型脂肪酸在菌株抵御逆境胁迫中的作用并未取得一致观点。如:郑昀昀等[21]在新物种Anoxybacillus flavithermus ssp. Yunnanesis E13T对甲苯的耐受性,以及Huang 等[11]对植物乳杆菌ZDY2013 对酸的耐受性研究中,均证实饱和脂肪酸发挥了积极作用,与本研究结果相一致;而Taranto等[22]在罗伊氏乳杆菌耐胆盐、袁峥[23]在嗜酸乳杆菌耐酸的相关研究中发现,不饱和脂肪酸比例增加对菌体耐受性更有利;由此可见,不同特性的菌株或不同因子胁迫下其耐受性机制也不尽相同。
综上,本研究发现了植物乳杆菌R23抗氧化酶、膜磷脂脂肪酸等在其应答二氧化硫引发的氧化应激中的作用,可初步阐明该菌对二氧化硫胁迫的适应性生理机制,为深入理解该菌抵抗高二氧化硫环境而生存的分子机制提供理论依据,同时为筛选高抗逆性菌株以解决目前高酸性果酒降酸中存在的问题提供思路。
-
表 1 正交因素及水平
Table 1 Orthogonal factors and levers
水平
LevelA.蔗糖
Sucrose /(g·L−1)B.H3BO3/
(g·L−1)C.Ca(NO3)2/
(g·L−1)D.PEG-4000/
(g·L−1)1 0 0 0 0 2 50 0.01 0.01 50 3 100 0.02 0.02 100 4 150 0.03 0.03 150 5 200 0.04 0.04 200 表 2 钦蜜9号花粉离体培养基筛选的L25(54)正交试验设计
Table 2 L25(54) orthogonal experimental design for culture medium of passion fruit pollens
编号
No.因素
Factors萌发率
Germination rate/%花粉管长度
Pollen tube length/μmA B C D 1 1 1 1 1 0 j — 2 1 2 2 2 13.81±1.88 i 158.5±4.03 ab 3 1 3 3 3 48.25±3.12 g 152.92±2.73 ab 4 1 4 4 4 64.50±1.68 f 126.24±5.96 e 5 1 5 5 5 75.53±3.07 bcde 108.29±10.29 f 6 2 1 2 3 69.11±11.13 def 147.43±4.46 bc 7 2 2 3 4 79.85±1.03 abc 132.59±8.86 de 8 2 3 4 5 63.65±4.68 f 109.89±5.34 f 9 2 4 5 1 12.99±2.23 i 150.65±13.17 b 10 2 5 1 2 72.26±1.83 cdef 167.15±4.46 a 11 3 1 3 5 45.95±0.61 g 143.76±7.77 bcd 12 3 2 4 1 0 j — 13 3 3 5 2 76.99±5.37 abcde 126.75±5.77 e 14 3 4 1 3 67.23±7.08 f 134.33±12.72 cde 15 3 5 2 4 85.56±1.82 a 157.36±4.01 ab 16 4 1 4 2 6.5±3.04 ij — 17 4 2 5 3 28.73±17.87 h 124.43±12.31 e 18 4 3 1 4 83.08±3.5 ab 120.2±3.76 ef 19 4 4 2 5 0.27±0.47 j — 20 4 5 3 1 0.18±0.17 j — 21 5 1 5 4 0.54±0.47 j — 22 5 2 1 5 1.03±0.4 j — 23 5 3 2 1 0 j — 24 5 4 3 2 1.66±2.88 j — 25 5 5 4 3 25.94±3.2 h 158.54±9.45 ab k1 40.42 24.42 44.72 2.63 k2 59.57 24.68 33.75 34.24 k3 41.64 54.40 34.53 47.85 k4 23.75 29.33 32.12 62.71 k5 5.83 51.89 38.96 37.29 R 53.74 29.98 12.60 60.08 数据表示为平均值±标准差。同列数据用不同小写字母标识表示在0.05水平差异显著。
Data are represented as mean ± standard deviation; those with different lowercase letters on same column indicate significant differences at P<0.05.表 3 钦蜜9号离体萌发率方差分析结果
Table 3 Variance analysis on in vitro germination rate of Qinmi 9
变异来源
Source of variationIII 类平方和
Class III sum of squares自由度
Degree of freedom均方
Mean squareF 显著性
Significance(P)蔗糖
Sources9986.344 4 2496.586 8.365 0.006** H3BO3 4465.681 4 1116.420 3.740 0.53 Ca(NO3)2 505.625 4 126.406 0.424 0.788 PEG-4000 9836.241 4 2459.06 8.239 0.006** 误差
Error2387.782 8 298.473 **表示在0.01水平差异极显著。
**indicates extremely significant difference at 0.01 level.表 4 不同方法检测的西番莲花粉活力
Table 4 Passion fruit pollen viability detected by different methods
种质
Germplasm染色率
Staining rate/%离体萌发率
In vitro
germination
rate/%I2-IK TTC 亚历山大
Alexandria台农
Tainong83.83±3.76 a 67.69±5.29 a 81.19±2.78 a 74.13±2.89 a 钦蜜9号
Qinmi 981.79±7.75 b 70.29±4.47 b 97.95±1.14 a 79.76±7.80 b 雅蜜
Yami87.85±3.22 b 69.97±5.92 c 95.42±2.16 a 63.71±2.84 c 白花
White Flowers84.32±7.73 b 77.41±6.33bc 97.22±1.89 a 63.7±1.00 c 蓝冠
Blue Crown80.41±3.26 a 64.85±4.41 b 78.50±5.28 a 60.79±4.61 b 香蜜
Xiangmi84.32±7.70 b 63.78±1.11 c 98.75±1.88 a 71.34±6.97 c 数据表示为平均值±标准差,同行数据后不同小写字母表示在0.05水平差异显著。
Data are mean±SD; those with different lowercase letters on same row indicate significant difference at 0.05 level. -
[1] DE SOUZA SILVA G, DA SILVA CAMPELO BORGES G, PINHO DA COSTA CASTRO C D, et al. Physicochemical quality, bioactive compounds and in vitro antioxidant activity of a new variety of passion fruit cv. BRS Sertão Forte (Passiflora cincinnata Mast. ) from Brazilian Semiarid region [J]. Scientia Horticulturae, 2020, 272: 109595. DOI: 10.1016/j.scienta.2020.109595
[2] GADIOLI I L, DE SÁ BARRETO DA CUNHA M, DE CARVALHO M V O, et al. A systematic review on phenolic compounds in Passiflora plants: Exploring biodiversity for food, nutrition, and popular medicine [J]. Critical Reviews in Food Science and Nutrition, 2018, 58(5): 785−807. DOI: 10.1080/10408398.2016.1224805
[3] DA SILVA LIMA L K, DE JESUS O N, SOARES T L, et al. Growth, physiological, anatomical and nutritional responses of two phenotypically distinct passion fruit species (Passiflora L. ) and their hybrid under saline conditions [J]. Scientia Horticulturae, 2020, 263: 109037. DOI: 10.1016/j.scienta.2019.109037
[4] XIA Z Q, HUANG D M, ZHANG S K, et al. Chromosome-scale genome assembly provides insights into the evolution and flavor synthesis of passion fruit (Passiflora edulis Sims) [J]. Horticulture Research, 2021, 8: 14. DOI: 10.1038/s41438-020-00455-1
[5] 罗远华, 陈燕, 方能炎, 等. 文心兰花粉离体萌发培养基的筛选及萌发率的测定 [J]. 福建农业学报, 2023, 38(11):1293−1301. LUO Y H, CHEN Y, FANG N Y, et al. Optimized medium and culture conditions for germination of Oncidium pollens [J]. Fujian Journal of Agricultural Sciences, 2023, 38(11): 1293−1301. (in Chinese)
[6] 田青兰, 吴艳艳, 张英俊, 等. 西番莲自交和异交的花粉管荧光显微观察及授粉亲和性分析 [J]. 果树学报, 2023, 40(1):98−110. TIAN Q L, WU Y Y, ZHANG Y J, et al. Pollen tube fluorescence microscopic observation and pollination compatibility analysis of self and cross-pollination in passion fruit(Passiflora edulis) [J]. Journal of Fruit Science, 2023, 40(1): 98−110. (in Chinese)
[7] FACCIUTO P G. Breeding Advances in Passiflora spp. (Passionflower) Native to Argentina [J]. Floriculture & Ornamental Biotechnology, 2011, 5(1):23-34.
[8] 白梦, 李博, 陈简村, 等. 报春苣苔属植物杂交亲和性分析及杂交障碍 [J]. 中南林业科技大学学报, 2024, 44(1):89−96. BAI M, LI B, CHEN J C, et al. Compatibility and crossbreeding barriers of hybridization in Primulina [J]. Journal of Central South University of Forestry & Technology, 2024, 44(1): 89−96. (in Chinese)
[9] 张波, 秦垦, 黄婷, 等. 宁夏枸杞花粉活力及柱头可授性研究 [J]. 湖北农业科学, 2022, 61(19):83−86. ZHANG B, QIN K, HUANG T, et al. Study on pollen viability and stigma receptivity of Lycium barbarum [J]. Hubei Agricultural Sciences, 2022, 61(19): 83−86. (in Chinese)
[10] 任少华, 张驰强, 王陈, 等. 香榧花粉活性检测及离体培养条件筛选 [J]. 种子, 2020, 39(8):104−108. REN S H, ZHANG C Q, WANG C, et al. Pollen viability detection and screening of in vitro culture conditions of torreyagrandis [J]. Seed, 2020, 39(8): 104−108. (in Chinese)
[11] 付强, 陈伟, 李江, 等. 思茅松花粉活力测定方法研究 [J]. 西部林业科学, 2020, 49(3):7−13. FU Q, CHEN W, LI J, et al. Selection of measurements on pollen viability in Pinus kesiya var. langbianensis [J]. Journal of West China Forestry Science, 2020, 49(3): 7−13. (in Chinese)
[12] 任飞艳, 陈显, 王士泉. 卵叶海桑花粉活力测定方法的比较研究 [J]. 种子, 2021, 40(3):35−39. REN F Y, CHEN X, WANG S Q. Comparative study on the determination methods of pollen viability of Sonneratia ovata [J]. Seed, 2021, 40(3): 35−39. (in Chinese)
[13] 张峥. 金叶含笑传粉生物学与杂交育种研究[D]. 长沙: 中南林业科技大学, 2023. ZHANG Z. Study on pollination biology and cross breeding of Michelia foveolata[D]. Changsha: Central South University of Forestry & Technology, 2023. (in Chinese)
[14] 刘洁云, 吴艳艳, 牟海飞, 等. 黄百香果花粉离体培养基筛选及贮藏试验 [J]. 西南农业学报, 2021, 34(8):1699−1704. LIU J Y, WU Y Y, MOU H F, et al. Medium screening for in vitro germination and storage conditions of yellow passion fruit pollen [J]. Southwest China Journal of Agricultural Sciences, 2021, 34(8): 1699−1704. (in Chinese)
[15] 吴斌, 黄东梅, 邢文婷, 等. 不同黄金百香果品种果肉挥发性成分分析 [J]. 中国果树, 2023, (9):57−64,143. WU B, HUANG D M, XING W T, et al. Analysis of volatile components in sarcocarp of different varieties of yellow passion fruit [J]. China Fruits, 2023(9): 57−64,143. (in Chinese)
[16] 王金花, 张健琴, 成良强, 等. 不同方法测定花生花粉活力的比较研究 [J]. 种子, 2022, 41(8):126−130. WANG J H, ZHANG J Q, CHENG L Q, et al. Comparative study on pollen vitality determination of peanut by different methods [J]. Seed, 2022, 41(8): 126−130. (in Chinese)
[17] 胡晋. 花粉的保存和生活力测定 [J]. 种子, 1992, 11(6):33−35,39. HU J. Preservation of pollen and determination of its viability [J]. Seed, 1992, 11(6): 33−35,39. (in Chinese)
[18] 刘帅, 彭方仁. 薄壳山核桃花粉活力测定方法比较 [J]. 经济林研究, 2023, 41(2):110−119. LIU S, PENG F R. Comparison of detection methods for pollen viability of Carya illinoinensis [J]. Non-wood Forest Research, 2023, 41(2): 110−119. (in Chinese)
[19] 刘蓉, 吴德军, 王因花, 等. 白蜡花粉最佳离体萌发培养基筛选 [J]. 南京林业大学学报(自然科学版), 2023, 47(3):70−76. LIU R, WU D J, WANG Y H, et al. Screening of optimal germination medium for in vitro Fraxinus [J]. Journal of Nanjing Forestry University (Natural Sciences Edition), 2023, 47(3): 70−76. (in Chinese)
[20] 刘禹廷, 蓝登明, 余伟莅, 等. 6种云杉属植物花粉活性测定 [J]. 安徽农业科学, 2015, 43(17):241−245. LIU Y T, LAN D M, YU W L, et al. The assay about the activity of six kinds of Picea pollen [J]. Journal of Anhui Agricultural Sciences, 2015, 43(17): 241−245. (in Chinese)
[21] 张晓宁, 叶航, 吴方圆, 等. 香花油茶无性系花粉离体萌发培养基及贮藏条件分析 [J]. 分子植物育种, 2023, (12):1−17 ZHANG X N, YE H, WU F Y, et al. In Vitro Germination of Camellia osmantha Pollen and lts StorageConditions Research [J]. Molecular Plant Breeding, 2023(12): 1−17. (in Chinese)
[22] DAVIDSON R. Davidson RE, ed. Handbook of water soluble gums and resins[M]. New York: McGraw-Hill, 1980: 1-18, 31.
[23] FERRARI T E, WALLACE D H. Germination of Brassica pollen and expression of incompatibility in vitro [J]. Euphytica, 1975, 24(3): 757−765. DOI: 10.1007/BF00132915
[24] ZHANG H Q, CROES A F. A new medium for pollen germination in vitro [J]. Acta Botanica Neerlandica, 1982, 31(1/2): 113−119.
[25] READ S M, CLARKE A E, BACIC A. Stimulation of growth of culturedNicotiana tabacum W38 pollen tubes by poly(ethylene glycol) and Cu(II) salts [J]. Protoplasma, 1993, 177(1): 1−14.
[26] 张超仪, 耿兴敏. 六种杜鹃花属植物花粉活力测定方法的比较研究 [J]. 植物科学学报, 2012, 30(1):92−99. DOI: 10.3724/SP.J.1142.2012.10092 ZHANG C Y, GENG X M. Comparative study on methods for testing pollen viability of the six species from genus Rhododendron [J]. Plant Science Journal, 2012, 30(1): 92−99. (in Chinese) DOI: 10.3724/SP.J.1142.2012.10092
[27] 张彬, 芮雯奕, 郑建初, 等. 水稻开花期花粉活力和结实率对高温的响应特征 [J]. 作物学报, 2007, 33(7):1177−1181. ZHANG B, RUI W Y, ZHENG J C, et al. Responses of pollen activity and seed setting of rice to high temperature of heading period [J]. Acta Agronomica Sinica, 2007, 33(7): 1177−1181. (in Chinese)
[28] 刘帮龙, 张晓慧, 干友民, 等. 野生马蹄金花粉生活力检测方法比较 [J]. 草业科学, 2011, 28(11):1941−1944. LIU B L, ZHANG X H, GAN Y M, et al. Detection methods of pollen viability of wild Dichondra repens [J]. Pratacultural Science, 2011, 28(11): 1941−1944. (in Chinese)
[29] 胡春, 刘左军, 李富香, 等. 钝裂银莲花花粉活力测定方法的研究 [J]. 植物研究, 2013, 33(5):582−586. DOI: 10.7525/j.issn.1673-5102.2013.05.014 HU C, LIU Z J, LI F X, et al. Detection methods for pollen viability of Anemone obtusiloba [J]. Bulletin of Botanical Research, 2013, 33(5): 582−586. (in Chinese) DOI: 10.7525/j.issn.1673-5102.2013.05.014
[30] 高露璐, 李林芳, 马育珠, 等. 毛叶铁线莲花粉活力测定方法 [J]. 分子植物育种, 2017, 15(11):4667−4672. GAO L L, LI L F, MA Y Z, et al. Measuring methods of Clematis lanuginose pollen viability [J]. Molecular Plant Breeding, 2017, 15(11): 4667−4672. (in Chinese)
[31] 蔡昭艳, 董龙, 王葫青, 等. 百香果花不同发育阶段花粉活力、柱头可授性及其对坐果的影响 [J]. 果树学报, 2023, 40(5):969−977. CAI Z Y, DONG L, WANG H Q, et al. Pollen viability, stigma receptivity and their effect on fruit set of passionfruit at different flower developmental stages [J]. Journal of Fruit Science, 2023, 40(5): 969−977. (in Chinese)




 下载:
下载: