Preparation and In Vitro Inhibitory Effects of Specific Tripple Anti-vibrio Egg Yolk Antibody
-
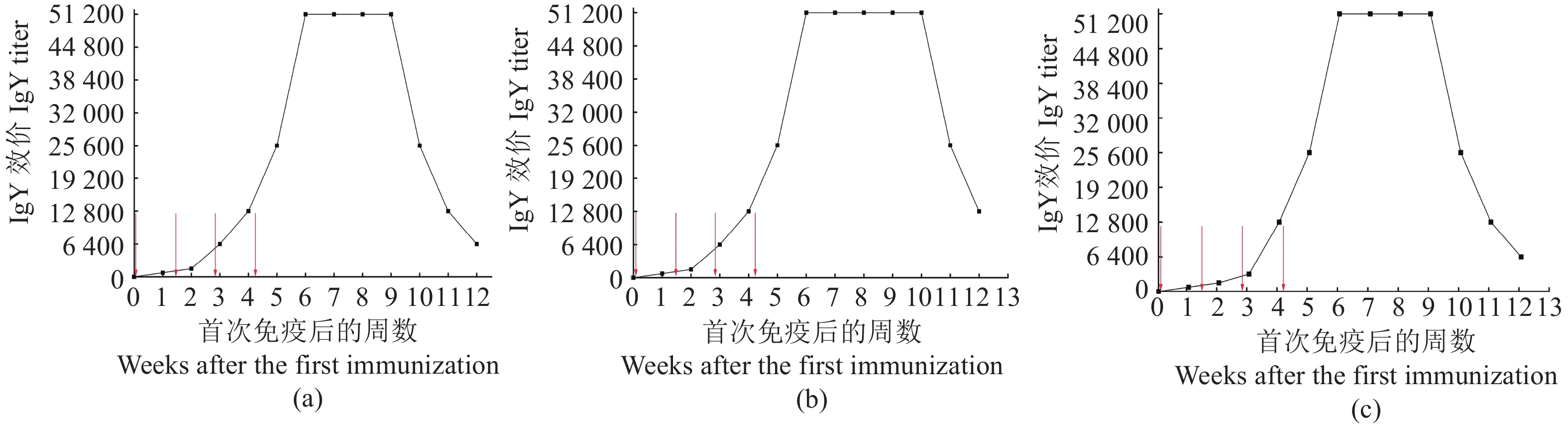
摘要:目的 制备抗弧菌三联特异性卵黄抗体,并研究其对创伤弧菌(Vibrio vulnificus)、河流弧菌(Vibrio fluvialis)和霍乱弧菌(Vibrio cholerae)的体外抑菌作用,为抗生素替代品的研发以及弧菌混合感染的防治提供参考。方法 制备创伤弧菌、河流弧菌和霍乱弧菌三联灭活疫苗,免疫蛋鸡,制备特异性卵黄抗体(IgY),并检测IgY的效价、纯度和特异性。通过免疫荧光、扫描电镜和体外抑菌试验初步探究制备的三联特异性卵黄抗体对3种弧菌的体外抑菌效果。结果 使用间接ELISA检测特异性IgY效价,分别以创伤弧菌、霍乱弧菌和河流弧菌作为抗原,特异性IgY效价峰值高达1∶
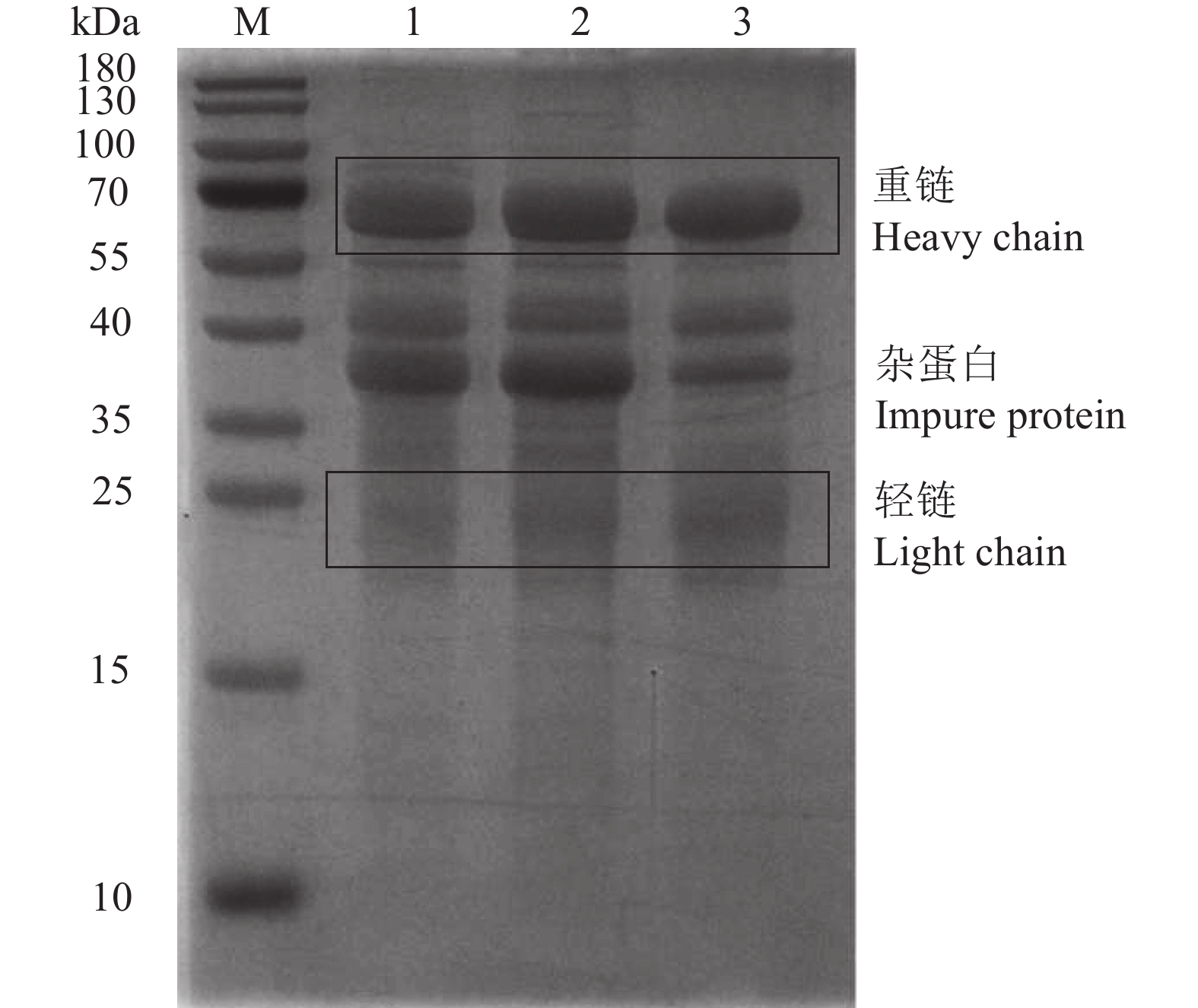
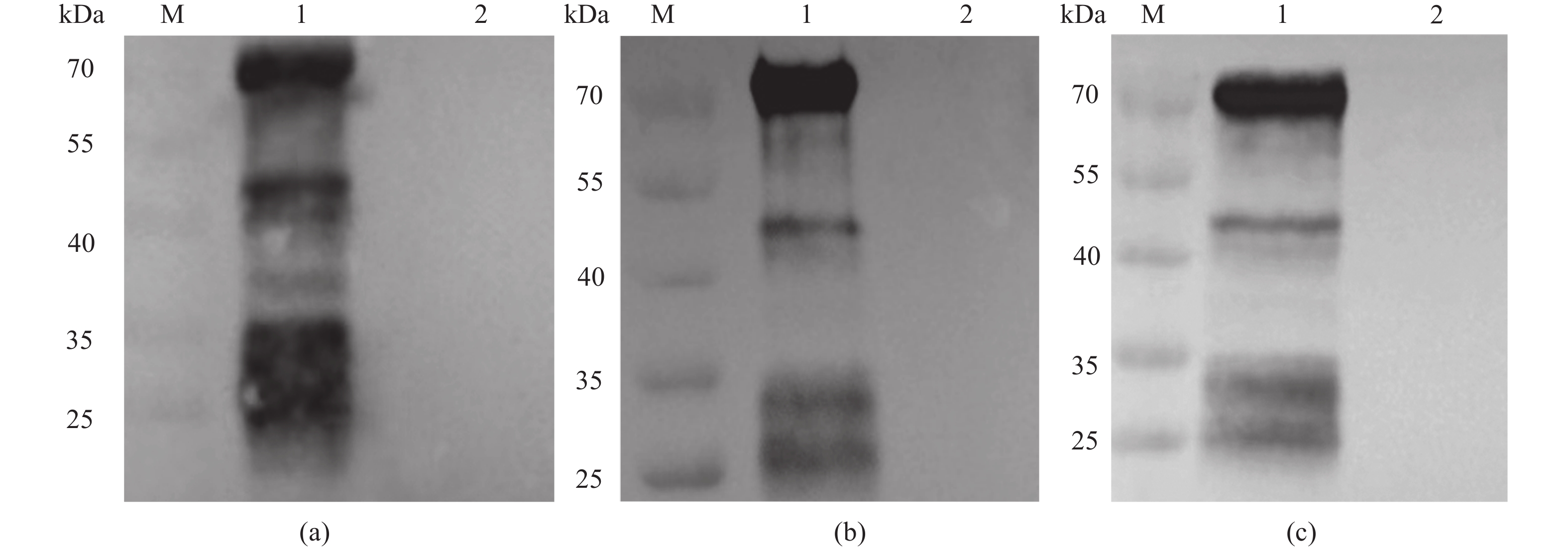
51200 ,可持续4~5周。对水稀释-盐析分离纯化的IgY进行SDS-PAGE 分析,可见IgY的重链和轻链,且蛋白杂带逐渐减少,纯度逐渐变高。免疫印迹分析发现,特异性IgY可以和抗原发生特异性结合,出现IgY的蛋白条带。免疫荧光试验结果表明,特异性IgY可以与3种弧菌发生特异性结合,出现明显的菌体凝集。扫描电镜观察发现,特异性IgY处理过的3种弧菌,菌体黏附,菌体细胞结构遭到破坏。体外抑菌试验发现,特异性IgY对3种弧菌的体外生长繁殖具有明显的抑制作用,抑菌效果随抗体用量增加而增强。结论 本研究制备的抗弧菌三联特异性卵黄抗体对创伤弧菌、河流弧菌和霍乱弧菌有较强的抑制作用,在弧菌混合感染的防治和开发新型抗生素替代品方面具有很大的潜力。Abstract:Objective In vitro inhibitory effects of the prepared specific anti-vibrio antibody on Vibrio vulnificus, V. fluvialis, and V. cholerae were studied for the development of a bioagent to prevent and treat infections caused by the viruses.Method Trivalent inactivated vaccine against the three specific viruses were prepared to immunize hens, and eggs collected for producing the specific IgY. Potency, purity, and specificity of the IgY were determined. Antiviral effects of the antibody were tested by immunofluorescence electron microscopy, scanning electron microscopy, and an in vitro test.Result Indirect ELISA detected the specific IgY with peak titers as high as 1:51200 that sustained for 4–5 weeks when the target viruses were used as antigens. The SDS-PAGE analysis on the IgY purified by means of water dilution and salt precipitation showed a gradual rise in purity, reduction in protein impurities, and increase in visible heavy and light bands. The immunoblotting of the specific IgY showed its binding to the corresponding antigens with appearance of the protein bands. The immunofluorescence electron microscope displayed apparent agglutination on the bindings to the three viruses. And furthermore, a scanning electron microscopic image of the viruses treated with the specific IgY detailed the adhesion of the antibody to and structural disruption of the virus cells. Finally, significant in vitro, dose-dependent inhibitory effects of the specific IgY on the growth and proliferation of target vibrio strains were observed.Conclusion The prepared specific triple anti-vibrio egg yolk antibody exhibited significant in vitro inhibitory effects on V. vulnificus, V. fluvialis, and V. cholerae. It seemed plausible that the application could become an effective alternative to antibiotic treatment in preventing and controlling the diseases caused by these viruses.-
Keywords:
- egg yolk antibody /
- Vibrio vulnificus /
- Vibrio fluvialis /
- Vibrio cholerae /
- inhibition
-
0. 引言
【研究意义】非洲菊(Gerbera jamesonii Bolus)隶属菊科(Asteraceae)大丁草属(Gerbera Cass.),为多年生宿根花卉,是世界五大鲜切花之一。目前已在国内的大部分地区种植,种植面积约4666.66 hm2[1]。福建省是非洲菊的主要产区之一,仅清流县种植规模就已超过400 hm2[2]。然而目前主栽品种仍为云南红、玲珑、紫灵等老品种,这些品种经多年种植,已经出现畸形花比例增多、花径变小、切花产量下降等品种衰退现象。因此选育综合性状优异、迎合市场需求的非洲菊新品种迫在眉睫。当前非洲菊新品种主要通过有性杂交及优株选择选育而得,而非洲菊为雌雄同花植物,其花序开放属雌蕊先熟型,自花授粉比较困难[3],同一无性系不同花朵间自交相对容易,自交结籽率可达46.9%[4],部分品种仍存在一定的自花授粉可育性,自交可育型品种的自花授粉可育性约为35%[5],可能造成杂交后代真假杂种混杂。而在非洲菊的遗传与育种研究中,获得具有双亲优良基因的真实杂交种是基础,为此对非洲菊杂种后代进行真实性鉴定十分必要。【前人研究进展】传统的杂种鉴定方法主要有形态学、细胞学及同工酶学等方法,鉴定周期长,鉴定结果易受环境条件影响。简单重复序列(Simple sequence repeat,SSR)分子标记具有共显性、多态性高、重复性好、简便易行等优点,广泛应用于石斛(Dendrobium Sw.)、马铃薯(Solanum tuberosum L.)、芒(Miscanthus sinensis Anderss.)等多种植物杂种F1的鉴定[6-8]。【本研究切入点】对于非洲菊SSR分子标记的研究主要集中在SSR位点分析[9,10]、遗传多样性分析[11-13]、基因定位[14]等方面,SSR标记应用于非洲菊杂种真实性鉴定的报道仍较为鲜见。【拟解决的关键问题】本研究以形态差异较大的浅色花心丝状花瓣非洲菊品种罗德里和深色花心舌状花瓣非洲菊品种热带草原及其正反交后代、部分自主选育非洲菊新品种(系)及其亲本为材料,通过从非洲菊转录组中发掘的65对SSR引物中筛选条带清晰、重复性好、亲本间无相同等位基因位点的引物,应用于非洲菊杂交后代及自主选育新品种(系)的杂种真实性鉴定,旨在明确SSR分子标记鉴定非洲菊杂交后代真实性的可行性,为提高非洲菊杂交育种效率及进一步开展非洲菊遗传研究奠定基础。
1. 材料与方法
1.1 试验材料
本试验所用的罗德里、热带草原等10个非洲菊杂交亲本均为三明市农业科学研究院花卉研究所从市场收集而来(表1),种质均保存于三明市农业科学研究院国家非洲菊种质资源库内,其花色、瓣型、花型等性状表现如图1所示。2021年11月以罗德里及热带草原为亲本进行正反交,2021年12月将正反交后代种子播种于穴盘内,并置于人工气候室培养,2022年4月将穴盘苗移栽至三明市农业科学研究院非洲菊种植大棚。2022年9月,从正反交后代中分别随机选取33个、32个单株,从自主选育非洲菊新品种(系)及其亲本中各选取1个单株(表2),剪取嫩叶样品,液氮冷冻后置于−80 ℃的超低温冰箱中保存备用。
表 1 亲本材料相关信息Table 1. Information about parent materials序号
Number种质名称
Germplasm name花色
Flower color瓣型
Petal type花型
Flower type花心颜色
Color of inflorescence center1 罗德里 Rodrigo 紫色 Purple 丝状 Spider 单瓣 Simple 浅色 Light 2 热带草原 Savannah 红色 Red 舌状 Ligulate 单瓣 Simple 深色 Dark 3 玲珑 Rosalin 粉色 Pink 舌状 Ligulate 半重瓣 Semidouble 深色 Dark 4 水粉 Ellymay 粉色 Pink 舌状 Ligulate 半重瓣 Semidouble 浅色 Light 5 红胜利 Hongshengli 红色 Red 舌状 Ligulate 半重瓣 Semidouble 深色 Dark 6 云南红 Yunnanhong 红色 Red 舌状 Ligulate 半重瓣 Semidouble 浅色 Light 7 拉丝6号 Spider No. 6 粉色 Pink 丝状 Spider 半重瓣 Semidouble 深色 Dark 8 拉丝4号 Spider No. 4 橙色 Orange 丝状 Spider 单瓣 Simple 深色 Dark 9 晨光 Chenguang 橙红复色 Orange and red 舌状 Ligulate 半重瓣 Semidouble 深色 Dark 10 菲比 Febe 橙黄复色 Orange and yellow 舌状 Ligulate 半重瓣 Semidouble 深色 Dark ![]() 图 1 10个非洲菊杂交亲本及4个非洲菊新品种(系)A:罗德里;B:热带草原;C:玲珑;D:水粉;E:红胜利;F:云南红;G:拉丝6号;H:拉丝4号;I:晨光;J:菲比;k:明卉粉黛;L:明卉红颜;M:魅粉;N:幻彩。Figure 1. Ten parents and 4 new cultivars/strains of gerberaA: Rodrigo; B: Savannah; C: Rosalin; D: Ellymay; E: Hongshengli; F: Yunnanhong; G: Spider No. 6; H: Spider No.4; I: Chenguang; J: Febe; K: Minghuifendai; L: Minghuihongyan; M: Meifen; N: Huancai.表 2 杂交亲本组合及其杂种F1代信息Table 2. Information on cross combinations and F1 hybrids
图 1 10个非洲菊杂交亲本及4个非洲菊新品种(系)A:罗德里;B:热带草原;C:玲珑;D:水粉;E:红胜利;F:云南红;G:拉丝6号;H:拉丝4号;I:晨光;J:菲比;k:明卉粉黛;L:明卉红颜;M:魅粉;N:幻彩。Figure 1. Ten parents and 4 new cultivars/strains of gerberaA: Rodrigo; B: Savannah; C: Rosalin; D: Ellymay; E: Hongshengli; F: Yunnanhong; G: Spider No. 6; H: Spider No.4; I: Chenguang; J: Febe; K: Minghuifendai; L: Minghuihongyan; M: Meifen; N: Huancai.表 2 杂交亲本组合及其杂种F1代信息Table 2. Information on cross combinations and F1 hybrids序号
Number杂交组合(♀×♂)
Hybrid combination (♀×♂)供试杂种F1代数量
Amount of tested F1 hybrids杂种F1编号/名称
F1 Hybrids number/cultivar1 罗德里×热带草原
Rodrigo×Savannah33 1、2、3、4、5、6、7、8、9、10、11、12、
13、14、15、16、17、18、19、20、21、22、23、24、
25、26、27、28、29、30、31、32、332 热带草原×罗德里
Savannah×Rodrigo32 34、35、36、37、38、39、40、41、42、43、44、
45、46、47、48、49、50、51、52、53、54、55、56、57、
58、59、60、61、62、63、64、653 玲珑×水粉
Rosalin×Ellymay1 LA14/明卉粉黛
LA14/Minghuifendai4 红胜利×云南红
Hongshengli×Yunnanhong1 50-101/明卉红颜
50-101/Minghuihongyan5 拉丝6号×拉丝4号
Spider No. 6×Spider No.41 F6-1/魅粉
F6-1/Meifen6 晨光×菲比
Chenguang×Febe1 CF-1/幻彩
CF-1/Huancai1.2 试验方法
1.2.1 DNA的提取和检测
用E.Z.N.A.TM HP Plant DNA Kit植物基因组提取试剂盒提取非洲菊基因组DNA,采用1%的琼脂糖凝胶电泳法检测DNA质量,使用BioDrop 超微量蛋白核酸分析仪测定DNA质量浓度,并将各DNA样品质量浓度稀释至20 ng·μL−1,放置于−20 ℃冰箱中保存备用。
1.2.2 SSR引物合成
从三明市农业科学研究院以非洲菊品种云南红转录组序列为基础开发的EST-SSR引物中[10],随机选取二核苷酸(20对)、三核苷酸(20对)、四核苷酸(14对)、五核苷酸(7对)、六核苷酸(4对)重复单元的SSR引物共65对(表3),引物由福州尚亚生物技术有限公司合成。
表 3 SSR引物序列Table 3. Sequence of SSR primers引物名称
Primer name重复单元
Repeat motif上游引物
Forward primer下游引物
Reverse primer片段长度
Fragment length/bpg01 (CT)14 GAATTCAATGAGCATCGCCT GGCGGGCAATACAAAACTTA 188 g02 (CT)12 TTCATTTCTCCCCTCGTCAC AATGGGTCAACATTCAGCGT 128 g03 (AC)11 GGATTTATTTGGTCTACGGTGC TTGGGAAGGGTTTGAAATTG 148 g04 (TG)18 TGCTAGGTGCTGTGAGGAGA TTGTGCACGCCTACTTTTTG 184 g05 (TC)11 TCCAATTCCAAGGTGTAAATCC GGAATTCTCCATTCCTGCAA 175 g06 (TA)8 CAAACGTCAAGAACACGCAC TCAACAGCGGTTGTGTATGAA 167 g07 (GA)15 GCGTAGGGTTTTCTGTGCAT TCTCTCTAAGATCGCCCTGC 206 g08 (TC)12 GCCAAGAAATGGATCCAAGA ACCCGCTCATTTTACGACC 134 g09 (AG)13 CGAACCTTCACAAGATCGGT TCGGAGATGTTCCTTTGACC 189 g10 (GT)12 GTGCGGGTGTGAACAACATA ATCACCTTCTCCGACACACC 159 g11 (GA)15 GTAGCGAAACACGGAGGAAA AGTACGGCCTCCTCCATTCT 194 g12 (GT)11 AACCTGGCATACACTTTGGC CGAACCAAACAATTACCATGAA 177 g13 (TC)12 GTTGCACGCCCTCCTATCT GTCGGTGTCGGAGAAATGTT 226 g14 (GA)9 TGCAATTGGATGTGAGTCGT GCAACGAGAGCAAACTACCC 179 g15 (TC)13 ACGGTTCAATTTCGAGAACG AAATTTTAGCGCAAAACAAGC 202 g16 (CT)9 GCTCTCAACCTGTCAAAGGC GCTTCCCTCGATTGTAGCTG 173 g17 (AG)10 TCCAACGTCAATTCCAATCA AACTCTGTCGTGGTGTCGGT 156 g18 (TA)12 CAATCATGGCTGCATTTCAC TTTTCCACGTCAAACCATCA 220 g19 (TA)10 GTGAGGTGCAAGAGGAAAGC TACCAGCAGAAGCAGACGAA 221 g20 (AG)10 ATTGCACCCTCGTTTTATGC TCTGCTGCATCTTCATGCTT 185 g21 (ATG)7 TGCCTTGAAAGTGACGATGA GCGTAAGATTCTCCACAGGG 223 g22 (TCT)12 CTCCATTTTGTAGCCAAGAGTG GCCACCACTACTGAGGCATT 163 g23 (CGG)8 CATCCCTTACGTTGGCACTT CACCCTTGAAACCCTCTCTG 167 g24 (GAT)8 AGTGGGAGAAGCTATGCCAA GGGTCGCCATAGCAAATAGA 187 g25 (TTA)8 GATTGGATGCTAGCTTTGCC GGGCATTTTGGACATTTGAT 162 g26 (GAA)5 AGAAGAGTCCGTGGTGGCTA GGTGACTTCGTCTTGAGGGA 185 g27 (AAG)13 AATCCTCAATGCCACCTTTG GAGGCAGGAATTGACTGGAA 160 g28 (TAA)7 CGTTTTACATGCAGCCTCAA CTTTGCTTCCTCTGCCTGAC 167 g29 (ACT)6 AACAATAGGATCAAACGCGG TCGGATTGAAGGTGAGAAGG 152 g30 (GAC)5 CACAAACCCTTGTAGCGGAT ACATTCTTCACCGGAGCAAC 150 g31 (AAG)9 ATCGGCTCAAGGTAAGGGAT GCTCAATGGCTTCAGACACA 186 g32 (GAT)11 ATTTTGAAGGGATTGGTGGG TCATGCCATATTCCCTCGAT 174 g33 (AAG)11 CAGGGGCAGTTAGGTTCAAA TAGAATTGGACCCGCTATGG 186 g34 (CAA)7 TGGCAATCGTGCTTGTTAAA CCCCAATTCTATTTGGGACT 158 g35 (TAC)7 GTCACACGTGGTCGCATATC ACAAATCGAACTTTGACCCG 194 g36 (AAC)9 AGCAAGATCAAAAGACCCGA CCTTTGTCGTCATAGCAATCAA 167 g37 (TCG)9 CGCCATTAAAGCCTTCTTTC GGAAGGCTTGTGTTGGTTGT 151 g38 (GCG)7 AATGGCAGCTACTGCGTCTT TCACCATTAACGGCTGATGA 158 g39 (AAT)12 ACAAAGAATCCGTCCACCAG GACCGTATTGGGCAGGTCTA 164 g40 (GAA)10 GAGGCGTTATCGGACTTTGA TTCTTCTTGGGACGTAACCG 168 g41 (TCTT)5 CGGTCACTGGGAAACTTCAT CGTCCTCAATAATTGCCGTT 199 g42 (TTGT)5 ACCCACTTGGCTTGGGTATT CTGCTGAGGCTTTCATCTCC 142 g43 (TACA)5 CGCAAAGTGTAAACTGAAGTGG CCCAGCTTGACTCATGGTTT 150 g44 (CTAT)8 TTAGGAGTGGAGTCGCTGCT CGAAAAGCTAGCAAATGGACA 200 g45 (AGGA)6 GGCGTCTTGTTTCTTTTTCG AGCTGGGACCTGGGAATACT 144 g46 (TTTA)6 TGTCCATAAAATGCGGTCAA TAAAAGCCCACCCTCAATCA 232 g47 (TGTT)5 GAAATCCGTGAAAGGTCGAA TGTACAAACCCACCTCCCTT 193 g48 (TCGA)5 AAGAAGCTCGGCCTCTGATT TACCTTCGCGGATTGTTTTC 204 g49 (TGTA)5 TACAACGGGTTATCCAAGCC GGTGCAAATACAAGGTTCGTG 190 g50 (TATG)5 TGGTTGGAAAAGTCATTCACTC TCAACACCGAACCGACAATA 143 g51 (TATT)8 AAATCTTTGATGAATGCGGC GAAGAATCCCAATTGAGCCA 186 g52 (TATG)5 GCCTCACCTGAAGACGGTAG TACATATGCGATTGGGCCTT 155 g53 (TCAA)5 CCGGTCACTCTCACATGCTA CCATCACAGACGACGAAAGA 148 g54 (ATTT)5 ATTAAAGAGTGTGCAGGCGG AAGCAACAACGTCGGAAAAT 153 g55 (TAAAA)5 AACGACTAGCGATTCCATGA TGTGGGATGTAACAAGGCAA 182 g56 (AAAAG)5 GAGTATTTGGAGCGAAAGCG TGAACACTTGTATCCGTCGC 119 g57 (ACCAA)5 CTCTTTCCTTTTCACCGCAC TTCGTCTAGATCTTCGCCGT 173 g58 (TAAAA)5 GGGTTCGTTTTGCATTTAACTC CAGGACCTTTGATTTTGGTCA 183 g59 (ACCCG)5 CTGCCGGAATCAAAATGAAT CTTTAATGGTGGCAATGGCT 166 g60 (CACCC)5 TGCTTACACTTCCGTGCAAC ATGTTAGCTCCAGTTGGGCA 189 g61 (AAAAG)5 CATGGATAAACCCGTTTTGG TTTTCTCTTTCTGTTTCGCCA 170 g62 (GTCAAA)6 GTCGCAAGAACTTCCAAAGC TCCACCGACTTTGACTTTCA 237 g63 (CGTCTT)6 TTGCAAATGCAAATCCAATC AAACAGCAGTGGTGGTTTCC 175 g64 (AATGGG)6 CGCTTCCTCCTACAACAAGC GTGTCCCCACCATTCAAGTT 163 g65 (TGCTCC)6 AGCTTGCCATGGTTATGGTC GGCTTAAAAGATCCCCAAGG 231 1.2.3 SSR反应体系、PCR扩增程序及电泳检测
SSR-PCR反应体系为10 μL,包括SSR上下游引物各0.5 μL,DNA模板0.8 μL,2×Taq MasterMix 5 μL,无菌水3.2 μL。PCR扩增程序为:94 ℃预变性4 min,94 ℃变性30 s,55 ℃退火30 s,72 ℃延伸30 s,共35个循环;72 ℃延伸7 min,最后4 ℃保存PCR扩增产物。电泳检测:扩增产物取1 μL,加至8%的聚丙烯酰胺凝胶点样孔中,以恒定电压120 V,在1×TAE缓冲液中电泳1.5~2.0 h,Ultra GelRed核酸染料染色20 min后,置于凝胶成像系统中观察结果并进行拍照及记录条带位置。
1.2.4 SSR引物筛选
分别以不同非洲菊亲本DNA为模板,用不同的SSR引物进行PCR扩增,筛选能扩增出条带清晰、重复性好、亲本间无相同等位基因位点的引物,用于下一步杂种F1的真实性鉴定。
1.2.5 杂种F1真实性鉴定
分别以双亲和杂种F1 DNA为模板,利用筛选出的SSR引物进行PCR扩增,扩增图谱中,具有父本或兼具有父母本特征条带的为真杂种,仅有母本特征条带的为假杂种。
2. 结果与分析
2.1 SSR引物筛选
利用65对SSR引物对非洲菊杂交亲本罗德里和热带草原的DNA进行PCR扩增,结果表明:在SSR位点处,共有57对引物扩增出条带;43对引物扩增条带较清晰,可作为非洲菊杂交后代真实性鉴定的筛选用引物对;26对引物具有多态性,多态性比例为40%;g24、g64这2对引物扩增双亲均表现为条带单一、清晰可辨、位点差异明显的纯合带型,即aa×bb型;g32引物扩增双亲均为互不相同的两条带的杂合带型,即ab×cd型,4条条带清晰可辨,位点差异明显;g38引物扩增母本罗德里为两条带的杂合带型,扩增父本热带草原为位点差异明显的单一条带的纯合带型,即ab×cc型,因此筛选g24、g32、g38、g64这4对引物用于非洲菊罗德里和热带草原杂交所获得的F1代杂种的真实性鉴定。部分SSR引物对亲本组合DNA的PCR扩增结果见图2。
2.2 非洲菊杂交后代的SSR鉴定结果
引物g24、g32、g38、g64对非洲菊亲本罗德里、热带草原及其65个正反交后代DNA的PCR扩增结果如图3所示。
![]() 图 3 g24引物(A)、g32引物(B)、g38引物(C)、g64引物(D)对非洲菊亲本罗德里、热带草原及65个正反交后代DNA的PCR扩增结果M:DNA Marker;L:罗德里;R:热带草原;1~33:非洲菊正交后代单株,34~65:非洲菊反交后代单株。Figure 3. PCR amplification results of primers g24 (A), g32 (B), g38 (C), and g64 (D) on DNA of G. jamesonii parents Rodrigo, Savannah, and 65 positive and negative progeniesM: DNA marker; L: Rodrigo; R: Savannah; 1-33:Positive cross gerbera plants; 34-65:Negative cross gerbera plants.
图 3 g24引物(A)、g32引物(B)、g38引物(C)、g64引物(D)对非洲菊亲本罗德里、热带草原及65个正反交后代DNA的PCR扩增结果M:DNA Marker;L:罗德里;R:热带草原;1~33:非洲菊正交后代单株,34~65:非洲菊反交后代单株。Figure 3. PCR amplification results of primers g24 (A), g32 (B), g38 (C), and g64 (D) on DNA of G. jamesonii parents Rodrigo, Savannah, and 65 positive and negative progeniesM: DNA marker; L: Rodrigo; R: Savannah; 1-33:Positive cross gerbera plants; 34-65:Negative cross gerbera plants.在罗德里×热带草原的正交后代中,g24、g64引物扩增亲本SSR位点基因型为aa×bb型,33个正交后代扩增出的SSR位点基因型均为ab型(图3-A,3-D);g32引物扩增亲本SSR位点基因型为ab×cd型,33个正交后代均扩增出包含父本和母本其中1条带的杂合带型,即ac、ad、bc或bd型(图3-B);g38引物扩增亲本SSR位点基因型为ab×cc型,33个正交后代均扩增出包含父本和母本其中1条带的杂合带型,即ac或bc型(图3-C)。4对引物扩增正交后代均包含双亲的各一条带,均将33个正交后代鉴定为真杂种,正交后代真杂种率达100%。
在热带草原×罗德里的反交后代中,g24、g64引物扩增亲本SSR位点基因型为aa×bb型,32个反交后代扩增出的SSR位点基因型均为ab型(图3-A、D);g32引物扩增亲本SSR位点基因型为ab×cd型,32个反交后代均扩增出包含父本和母本其中1条带的杂合带型,即ac、ad、bc或bd型(图3-B);g38引物扩增亲本SSR位点基因型为aa×bc型,32个反交后代均扩增出包含母本和父本其中1条带的杂合带型,即ab或ac型(图3-C)。4对引物扩增反交后代均包含双亲的各一条带,均将32个反交后代鉴定为真杂种,反交后代真杂种率达100%。
2.3 非洲菊新品种(系)SSR标记鉴定
4个不同类型非洲菊新品种(系)及其利用双亲筛选出的鉴定引物如表4所示,鉴定结果如图4所示。在SSR位点处,g24引物扩增亲本玲珑、水粉,g04引物扩增亲本红胜利、云南红的基因型为aa×bb型,非洲菊新品种明卉粉黛、新品系明卉红颜基因型为ab型,均鉴定为真杂种;g44引物扩增亲本拉丝6号、拉丝4号,g39引物扩增亲本晨光、菲比基因型为ab×cc型,非洲菊新品系魅粉、幻彩均为含双亲各一条带的杂合带型,即ac型,均鉴定为真杂种。引物g24、g04、g44、g39扩增杂交后代均包含双亲的各一条带,分别将非洲菊新品种(系)明卉粉黛、明卉红颜、魅粉、幻彩鉴定为真杂种。
表 4 非洲菊新品种(系)及鉴定引物Table 4. SSR primers for identifying new gerbera cultivars/strains品种(系)名称
Cultivar/Strain材料
Material母本
Female parent父本
Male parent鉴定引物
Primer for identification明卉粉黛
Minghuifendai粉色花瓣、浅色花心非洲菊新品种
New cultivar with pink petal, light color in the center of inflorescence玲珑
Rosalin水粉
Ellymayg24 明卉红颜
Minghuihongyan红色花瓣、深色花心非洲菊新品系
New strains with red petal, dark color in the center of inflorescence红胜利
Hongshengli云南红
Yunnanhongg04 魅粉
Meifen粉色花瓣、深色花心、拉丝非洲菊新品系
New spider strains with pink petal, dark color in the center of inflorescence拉丝6号
Spider NO. 6拉丝4号
Spider NO. 4g44 幻彩
Huancai橙黄复色花瓣、深色花心非洲菊新品系
New strains with orange and yellow petal, dark color in the center of inflorescence晨光
Chenguang菲比
Febeg39 ![]() 图 4 基于SSR标记的非洲菊新品种(系)杂种真实性鉴定结果M:DNA marker,♀为母本,♂为父本,F1为杂交后代新品种(系),红色框部分所示为SSR位点处的差异条带;A:g24引物扩增电泳图,母本为玲珑,父本为水粉;B:g04引物扩增电泳图,母本为红胜利,父本为云南红;C:g44引物扩增电泳图,母本为拉丝6号,父本为拉丝4号;D:g39引物扩增电泳图,母本为晨光,父本为菲比Figure 4. Identification results of SSR markers in new gerbera cultivars/strainsM: DNA marker; ♀: Female parent; ♂: Male parent; F1: New cultivars/strains of hybrids; Red box shows different band at SSR position; A: Amplification electrophoresis on g24 of Rosalin as female parent and Ellymay as male parent; B: Amplification electrophoresis on g04 of Hongshengli as female parent and Yunnanhong as male parent; C: Amplification electrophoresis on g44 of Spider NO.6 as female parent and Spider NO.4 as male parent; D: Amplification electrophoresis on g39 of Chenguang as female parent and Febe as male parent.
图 4 基于SSR标记的非洲菊新品种(系)杂种真实性鉴定结果M:DNA marker,♀为母本,♂为父本,F1为杂交后代新品种(系),红色框部分所示为SSR位点处的差异条带;A:g24引物扩增电泳图,母本为玲珑,父本为水粉;B:g04引物扩增电泳图,母本为红胜利,父本为云南红;C:g44引物扩增电泳图,母本为拉丝6号,父本为拉丝4号;D:g39引物扩增电泳图,母本为晨光,父本为菲比Figure 4. Identification results of SSR markers in new gerbera cultivars/strainsM: DNA marker; ♀: Female parent; ♂: Male parent; F1: New cultivars/strains of hybrids; Red box shows different band at SSR position; A: Amplification electrophoresis on g24 of Rosalin as female parent and Ellymay as male parent; B: Amplification electrophoresis on g04 of Hongshengli as female parent and Yunnanhong as male parent; C: Amplification electrophoresis on g44 of Spider NO.6 as female parent and Spider NO.4 as male parent; D: Amplification electrophoresis on g39 of Chenguang as female parent and Febe as male parent.3. 讨论与结论
植物杂交后代的鉴定方法主要有形态学、细胞学、同工酶学以及分子标记等方法,但在实际应用中多采用简单的形态学鉴定,存在周期长、效率低等问题[15]。非洲菊因异花授粉和栽培种依靠组培繁殖,其栽培品种基因型高度杂合,杂交后代的观赏性状出现广泛分离。且由于非洲菊染色体数目多(2n=2X=50),研究其花色、瓣型、花心颜色等的遗传规律也十分困难[16],因此通过形态学的方法难以准确鉴定非洲菊的杂种后代。DNA分子标记技术在DNA水平上揭示了亲本与子代的遗传差异,具有检测快速、准确的特点,广泛应用于杂种的鉴定。目前应用于杂种鉴定的分子标记主要有SRAP[17]、RAPD[18]、SSR[19]、AFLP[19]等,其中SSR标记为共显性标记,能够区分杂合位点和纯合位点,重复性和稳定性均较好,操作简单,是杂种后代分子鉴定的常用手段[20]。相比于RAPD、AFLP显性标记,SSR标记的鉴定效率高[21],与同为共显性标记的SRAP标记相比,其扩增的谱带少、易于识别和统计[22],并已在文心兰(Oncidium flexuosum Lodd.)、卷丹百合(Lilium lancifolium Thunb.)、月季(Rosa chinensis Jacq.)等多种园艺作物中广泛应用[23-25]。本研究通过对65对SSR引物的筛选,共获得26对在非洲菊亲本间具有多态性的引物,多态性比例达40%。李永清等[6]的研究表明SSR引物在石斛亲本间多态性比例占38.1%,李文秀等[21]的研究结果显示SSR引物在橡胶树(Hevea brasiliensis Muell.Arg.)亲本间多态性频率为31.73%,本研究结果与李永清等[6]的研究结果相近,但高于李文秀等[21]的研究结果,这可能与植物基因组杂合度的差异有关。
亲本间等位基因多态性的不同SSR标记的杂种鉴定能力亦不相同,若亲本间无相同等位基因,理论上只需1个标记就可鉴定全部的杂种后代[25,26]。周宁宁等[25]的研究结果显示在二倍体月季亲本中,基因型为aa×bb型的3个纯合显性SSR标记,其杂交后代基因型均为ab型,3个纯合显性SSR标记的鉴定效率均达100%;张婧等[27]、朱骏驰等[15]的研究结果表明亲本基因型为ab×cd的SSR标记可分别准确鉴定柳枝稷(Panicum virgatum L.)与葡萄(Vitis vinifera L.)的F1代杂种;苏聪聪等[20]用亲本基因型为aa×bb的2对SSR引物准确鉴定了刺葡萄(Vitis davidii Foex.)的F1代杂种。本研究中,g24及g64引物扩增亲本基因型为aa×bb型,g32引物扩增亲本基因型为ab×cd型,g38引物扩增亲本基因型为ab×cc型,亲本间无相同等位基因位点,非洲菊杂交后代均包含双亲的各一条带,均准确鉴定了非洲菊的F1代杂种的真实性,单一引物鉴定效率均达100%,这与周宁宁[25]、张婧[27]、朱骏驰[15]、苏聪聪[20]等的研究结果一致,与理论结果一致[25,26]。此外,本研究未见仅有父本带型的真杂种,未见染色体异常植株,与苏聪聪等[20]、周宁宁等[25]的研究结果不一致,这可能与物种的差异有关。
在非洲菊杂种F1的真实性鉴定过程中,本研究通过对基于非洲菊转录组序列为基础开发的65对SSR引物的筛选,共获得在非洲菊亲本罗德里,热带草原间具有多态性的引物26对,多态性比例达40%;筛选出亲本间无相同等位基因位点的引物4对,均将65个非洲菊杂交F1代杂种鉴定为真杂种,单一引物鉴定效率达100%,表明SSR标记在不同非洲菊亲本间多态性丰富、杂种鉴定效率高。同时,本研究将SSR标记成功应用于常规舌状花瓣与丝状花瓣,浅色花心与深色花心,单色花和复色花等不同类型非洲菊种质杂交所获得的新品种(系)的杂种真实性鉴定中,分别只用1对引物就将新品种(系)进行了鉴定,进一步说明了SSR标记在非洲菊杂种鉴定中具有高效性。SSR分子标记在非洲菊育种中的应用,加速了非洲菊新品种的选育进程,为进一步开展非洲菊的遗传研究奠定基础。
-
图 4 特异性IgY对菌体细胞形态结构的影响
1、3、5:特异性IgY处理组;2、4、6:非特异性IgY处理组。1~2:创伤弧菌;3~4:河流弧菌;5~6:霍乱弧菌。
Figure 4. Effect of specific IgY on morphological structure of virus cells
1, 3, and 5: Specific IgY treatment group; 2, 4, and 6: non-treatment group; 1 and 2: V. vulnificus;3 and 4: V. fluvialis;5 and 6: V. cholerae.
图 5 免疫荧光电镜观察特异性IgY 与致病菌的结合情况
1、4、7:特异性IgY处理组;2、5、8:非特异性IgY处理组;3、6、9:空白对照组。1~3:创伤弧菌;4~6:河流弧菌;7~9:霍乱弧菌。
Figure 5. Binding of specific IgY to pathogenic vibrio observed under immunofluorescence electron microscope
1,4, and 7: specific IgY treatment group; 2, 5, and 8: non-treatment group; 3, 6, and 9: blank control group; 1–3: V. vulnificus;4–6: V. fluvialis;7–9: V. cholerae.
图 6 特异性IgY对致病菌体外生长抑制效果
a:创伤弧菌;b:河流弧菌;c:霍乱弧菌。数据以mean ± SD ( n = 3) 表示;***:表示同一时间不同浓度特异性IgY与不加IgY的空白组之间差异显著(P < 0.001)。
Figure 6. In vitro inhibitory effect of specific IgY on growth of pathogenic vibrio
a: V. vulnificus; b: V. fluvialis; c: V. cholerae. Data are presented as means ± SD (n = 3); ***: significant difference between different concentrations of specific IgY and blank without IgY at same time (P<0.001)。
-
[1] 邓益琴. 水产动物弧菌病及其生物防治研究进展 [J]. 大连海洋大学学报, 2023, 38(4):553−563. DENG Y Q. Progress in research on vibriosis and biological control in animals in aquaculture: A review [J]. Journal of Dalian Ocean University, 2023, 38(4): 553−563. (in Chinese)
[2] ELGENDY M Y, ABDELSALAM M, KENAWY A M, et al. Vibriosis outbreaks in farmed Nile Tilapia (Oreochromis niloticus) caused by Vibrio mimicus and V. cholerae [J]. Aquaculture International, 2022, 30(5): 2661−2677. DOI: 10.1007/s10499-022-00921-8
[3] SONY M, SUMITHRA T G, ANUSREE V N, et al. Antimicrobial resistance and virulence characteristics of Vibrio vulnificus, Vibrio parahaemolyticus and Vibrio harveyi from natural disease outbreaks of marine/estuarine fishes [J]. Aquaculture, 2021, 539: 736608. DOI: 10.1016/j.aquaculture.2021.736608
[4] 朱苏琴, 纪荣兴, 苏永全, 等. 河流弧菌(Vibrio fluvialis)对大黄鱼(Pseudosciaena crocea)鳃黏液黏附特性研究 [J]. 海洋与湖沼, 2012, 43(2):389−393. DOI: 10.11693/hyhz201202030030 ZHU S Q, JI R X, SU Y Q, et al. Study on adhesion characteristics of Vibrio fluvialis to the gill mucus of Pseudosciaena crocea [J]. Oceanologia et Limnologia Sinica, 2012, 43(2): 389−393. (in Chinese) DOI: 10.11693/hyhz201202030030
[5] URBAN-CHMIEL R, MAREK A, STĘPIEŃ-PYŚNIAK D, et al. Antibiotic resistance in bacteria-a review [J]. Antibiotics, 2022, 11(8): 1079. DOI: 10.3390/antibiotics11081079
[6] ZHANG W W, LI C H, GUO M. Use of ecofriendly alternatives for the control of bacterial infection in aquaculture of sea cucumber Apostichopus japonicus [J]. Aquaculture, 2021, 545: 737185. DOI: 10.1016/j.aquaculture.2021.737185
[7] CAKIR-KOC R. Production of anti-SAG1 IgY antibody against Toxoplasma gondii parasites and evaluation of antibody activity by ELISA method [J]. Parasitology Research, 2016, 115(8): 2947−2952. DOI: 10.1007/s00436-016-5047-9
[8] ZHANG L P, LIN L, QIN Z D. A review on the application of chicken immunoglobulin Y in aquaculture [J]. Reviews in Aquaculture, 2024, 16(1): 536−551. DOI: 10.1111/raq.12850
[9] EL-KAFRAWY S A, ABBAS A T, OELKRUG C, et al. IgY antibodies: The promising potential to overcome antibiotic resistance [J]. Frontiers in Immunology, 2023, 14: 1065353. DOI: 10.3389/fimmu.2023.1065353
[10] PEREIRA E P V, VAN TILBURG M F, FLOREAN E O P T, et al. Egg yolk antibodies (IgY) and their applications in human and veterinary health: A review [J]. International Immunopharmacology, 2019, 73: 293−303. DOI: 10.1016/j.intimp.2019.05.015
[11] GADDE U, RATHINAM T, LILLEHOJ H S. Passive immunization with hyperimmune egg-yolk IgY as prophylaxis and therapy for poultry diseases: A review [J]. Animal Health Research Reviews, 2015, 16(2): 163−176. DOI: 10.1017/S1466252315000195
[12] HUSSEIN M A, REHAN I F, REHAN A F, et al. Egg yolk IgY: A novel trend of feed additives to limit drugs and to improve poultry meat quality [J]. Frontiers in Veterinary Science, 2020, 7: 350. DOI: 10.3389/fvets.2020.00350
[13] REHAN I F, REHAN A F, ABOUELNAGA A F, et al. Impact of dietary egg yolk IgY powder on behavior, meat quality, physiology, and intestinal Escherichia coli colonization of broiler chicks [J]. Frontiers in Veterinary Science, 2022, 9: 783094. DOI: 10.3389/fvets.2022.783094
[14] GAO X J, ZHANG X J, SUN J J, et al. Passive protection effect of anti-Vibrio anguillarum IgY-encapsulated feed on half-smooth tongue sole (Cynoglossus semilaevi ) against V. anguillarum [J]. Fish & Shellfish Immunology, 2016, 56: 483−488.
[15] THOMSEN K, CHRISTOPHERSEN L, BJARNSHOLT T, et al. Anti-Pseudomonas aeruginosa IgY antibodies augment bacterial clearance in a murine pneumonia model [J]. Journal of Cystic Fibrosis, 2016, 15(2): 171−178. DOI: 10.1016/j.jcf.2015.08.002
[16] GUIMARÃES M C C, AMARAL L G, RANGEL L B A, et al. Growth inhibition of Staphylococcus aureus by chicken egg yolk antibodies [J]. Archivum Immunologiae et Therapiae Experimentalis, 2009, 57(5): 377−382. DOI: 10.1007/s00005-009-0041-x
[17] GAO X J, ZHANG X J, LIN L, et al. Passive Immune-Protection of Litopenaeus vannamei against Vibrio harveyi and Vibrio parahaemolyticus Infections with Anti-Vibrio Egg Yolk (IgY)-Encapsulated Feed [J]. International Journal of Molecular Sciences, 2016, 17(5): 723. DOI: 10.3390/ijms17050723
[18] YI L Z, QIN Z D, LIN H Z, et al. Features of chicken egg yolk immunoglobulin (IgY) against the infection of red-spotted grouper nervous necrosis virus [J]. Fish & Shellfish Immunology, 2018, 80: 534−539.
[19] 胡青青, 赵肃清, 贺攀, 等. 抗须癣毛癣菌细胞壁蛋白的卵黄抗体制备和鉴定 [J]. 中国免疫学杂志, 2017, 33(9):1350−1354. DOI: 10.3969/j.issn.1000-484X.2017.09.015 HU Q Q, ZHAO S Q, HE P, et al. Preparation and identification of specific chicken egg yolk immunoglobulins against cell wall protein of Trichophyton mentagrophytes [J]. Chinese Journal of Immunology, 2017, 33(9): 1350−1354. (in Chinese) DOI: 10.3969/j.issn.1000-484X.2017.09.015
[20] 翟玥, 曲笑锋, 庞博, 等. 副溶血性弧菌高免卵黄抗体的制备和不同提纯方法效果的比较 [J]. 吉林大学学报(医学版), 2017, 43(2):441−445. ZHAI Y, QU X F, PANG B, et al. Preparation of high immunity yolk antibody against Vibrio parahemolyticus and comparison of effectiveness between different extraction methods [J]. Journal of Jilin University (Medicine Edition), 2017, 43(2): 441−445. (in Chinese)
[21] 姜肖军, 郭亚男, 陈秀红, 等. 鸡滑液囊支原体卵黄抗体的制备与鉴定 [J]. 中国兽医科学, 2020, 50(8):1018−1022. JIANG X J, GUO Y N, CHEN X H, et al. The production and identification of eeg yalk antibody(IgY) against Mycoplasma synoviae [J]. Chinese Veterinary Science, 2020, 50(8): 1018−1022. (in Chinese)
[22] XU L, XU Y P, HE L Y, et al. Immunomodulatory effects of chicken egg yolk antibodies (IgY) against experimental Shewanella marisflavi AP629 infections in sea cucumbers (Apostichopus japonicus) [J]. Fish & Shellfish Immunology, 2019, 84: 108−119.
[23] XU L, CHE J, XU Y P, et al. Oral administration of microencapsulated egg yolk immunoglobulin (IgY) in turbot (Scophthalmus maximus) to combat against Edwardsiella tarda 2CDM001 infections [J]. Fish & Shellfish Immunology, 2020, 106: 609−620.
[24] QIN Z D, BABU V S, LI N Q, et al. Protective effects of chicken egg yolk immunoglobulins (IgY) against experimental Aeromonas hydrophila infection in blunt snout bream (Megalobrama amblycephala) [J]. Fish & Shellfish Immunology, 2018, 78: 26−34.
[25] 安芳兰, 李菁, 张荣, 等. 卵黄抗体在疾病防治中应用的研究进展 [J]. 安徽农业科学, 2016, 44(5):68−71. DOI: 10.3969/j.issn.0517-6611.2016.05.026 AN F L, LI J, ZHANG R, et al. Research progress of egg yolk antibody in the prevention and treatment of various diseases [J]. Journal of Anhui Agricultural Sciences, 2016, 44(5): 68−71. (in Chinese) DOI: 10.3969/j.issn.0517-6611.2016.05.026
[26] TSUBOKURA K, BERNDTSON E, BOGSTEDT A, et al. Oral administration of antibodies as prophylaxis and therapy in Campylobacter jejuni-infected chickens [J]. Clinical and Experimental Immunology, 1997, 108(3): 451−455.
[27] AUZUREEN A M Z, MICHAEL M S, MOHAMED M, et al. Detection of pathogenic Vibrio species and antibiogram activity in Asian Seabass (Lates calcarifer) in Tumpat, Kelantan [J]. Tropical Biomedicine, 2022, 39(4): 569−574. DOI: 10.47665/tb.39.4.013
[28] BAO J J, GUO D K, JIN L, et al. Accurate identification of diverse N-acyl homoserine lactones in marine Vibrio fluvialis by UHPLC-MS/MS [J]. Current Microbiology, 2022, 79(6): 181. DOI: 10.1007/s00284-022-02879-5
[29] CHEN Y H, AI X H, YANG Y B. Vibrio cholerae: A pathogen shared by human and aquatic animals [J]. The Lancet Microbe, 2022, 3(6): e402. DOI: 10.1016/S2666-5247(22)00125-2
[30] XIAO X X, LIN Z Q, HUANG X H, et al. Rapid and sensitive detection of Vibrio vulnificus using CRISPR/Cas12a combined with a recombinase-aided amplification assay [J]. Frontiers in Microbiology, 2021, 12: 767315. DOI: 10.3389/fmicb.2021.767315
[31] LIANG J F, PENG C, LI P Y, et al. A review of detection of antibiotic residues in food by surface-enhanced Raman spectroscopy [J]. Bioinorganic Chemistry and Applications, 2021, 2021: 8180154.
[32] DOU L N, ZHANG Y J, BAI Y C, et al. Advances in chicken IgY-based immunoassays for the detection of chemical and biological hazards in food samples [J]. Journal of Agricultural and Food Chemistry, 2022, 70(4): 976−991. DOI: 10.1021/acs.jafc.1c06750
[33] FISHMAN J B, BERG E A. Isolation of IgY from chicken eggs[J]. Cold Spring Harbor Protocols, 2018, 2018(6): pdb. prot099150.
[34] KARACHALIOU C E, VASSILAKOPOULOU V, LIVANIOU E. IgY technology: Methods for developing and evaluating avian immunoglobulins for the in vitro detection of biomolecules [J]. World Journal of Methodology, 2021, 11(5): 243−262. DOI: 10.5662/wjm.v11.i5.243
[35] KOVACS-NOLAN J, PHILLIPS M, MINE Y. Advances in the value of eggs and egg components for human health [J]. Journal of Agricultural and Food Chemistry, 2005, 53(22): 8421−8431. DOI: 10.1021/jf050964f
[36] MINE Y, KOVACS-NOLAN J. Biologically active hen egg components in human health and disease [J]. The Journal of Poultry Science, 2004, 41(1): 1−29. DOI: 10.2141/jpsa.41.1
[37] CAI R Z, LIU N, GUO P H, et al. Protective effects of chicken egg yolk immunoglobulins (IgYs) against Vibrio vulnificus infections [J]. Journal of Immunology Research, 2021, 2021: 6678513.
[38] RANJBAR M, BEHROUZ B, NOROUZI F, et al. Anti-PcrV IgY antibodies protect against Pseudomonas aeruginosa infection in both acute pneumonia and burn wound models [J]. Molecular Immunology, 2019, 116: 98−105. DOI: 10.1016/j.molimm.2019.10.005
[39] SHI H Y, ZHU J, ZOU B Y, et al. Effects of specific egg yolk immunoglobulin on pan-drug-resistant Acinetobacter baumannii [J]. Biomedicine & Pharmacotherapy, 2017, 95: 1734−1742.
[40] 张文平, 谢水祥, 傅颖媛, 等. 抗白念珠菌卵黄免疫球蛋白对白念珠菌生长及黏附的影响 [J]. 临床皮肤科杂志, 2005, 34(12):814−815. DOI: 10.3969/j.issn.1000-4963.2005.12.009 ZHANG W P, XIE S X, FU Y Y, et al. Effect of anti-Candida albicans yolk immunoglobulin on the growth and adhesion of Candida albicans [J]. Journal of Clinical Dermatology, 2005, 34(12): 814−815. (in Chinese) DOI: 10.3969/j.issn.1000-4963.2005.12.009
[41] 贺维朝, 张会艳, 王浩, 等. 卵黄抗体提取方法及其在畜禽细菌性肠道疾病防治中的应用 [J]. 中国畜牧兽医, 2021, 48(2):640−649. HE W Z, ZHANG H Y, WANG H, et al. Extraction methods of yolk antibody and its application in prevention and treatment of bacterial intestinal diseases in livestock and poultry [J]. China Animal Husbandry & Veterinary Medicine, 2021, 48(2): 640−649. (in Chinese)
[42] 陈翠萍, 杨朝晖, 王永谦. IgY抗体在体外和体内对幽门螺杆菌作用的研究 [J]. 中华微生物学和免疫学杂志, 2002, 22(1):37−40. DOI: 10.3760/j:issn:0254-5101.2002.01.013 CHEN C P, YANG Z H, WANG Y Q. Neutralization of cytotoxic activity of Helicobacter pylori and treatment of mice by specific IgY Antibody [J]. Chinese Journal of Microbiology and Immunology, 2002, 22(1): 37−40. (in Chinese) DOI: 10.3760/j:issn:0254-5101.2002.01.013
[43] KOTA R K, REDDY P N, SREERAMA K. Application of IgY antibodies against staphylococcal protein A (SpA) of Staphylococcus aureus for detection and prophylactic functions [J]. Applied Microbiology and Biotechnology, 2020, 104(21): 9387−9398. DOI: 10.1007/s00253-020-10912-5
[44] 姚羽菲, 龙敏, 文荟淋, 等. 抗铜绿假单胞菌IgY的制备优化及抑菌效果 [J]. 检验医学与临床, 2018, 15(2):202−205,208. DOI: 10.3969/j.issn.1672-9455.2018.02.018 YAO Y F, LONG M, WEN H L, et al. The optimized preparation of IgY against p. aeruginosa and the detection of its antibiotic effect [J]. Laboratory Medicine and Clinic, 2018, 15(2): 202−205,208. (in Chinese) DOI: 10.3969/j.issn.1672-9455.2018.02.018
[45] WANG Z B, LI J, LI J Z, et al. Protective effect of chicken egg yolk immunoglobulins (IgY) against enterotoxigenic Escherichia coli K88 adhesion in weaned piglets [J]. BMC Veterinary Research, 2019, 15(1): 234. DOI: 10.1186/s12917-019-1958-x
[46] KASSIM N, MTENGA A B, SHIM W B, et al. The in vitro and in vivo efficacy of hen IgY against Vibrio parahaemolyticus and Vibrio vulnificus [J]. Journal of Microbiology and Biotechnology, 2012, 22(10): 1423−1431. DOI: 10.4014/jmb.1204.04006
[47] ZHANG M X, GENG H J, TARIQ JAVED M, et al. Passive protection of Japanese pufferfish (Takifugu rubripes) against Vibrio harveyi infection using chicken egg yolk immunoglobulins (IgY) [J]. Aquaculture, 2021, 532: 736009. DOI: 10.1016/j.aquaculture.2020.736009
-
期刊类型引用(5)
1. 杜俊峰,袁瑗,邓春莉,任羽. 运用SRAP和SSR分子标记鉴定铁皮石斛与金钗石斛F1代杂种的真实性. 现代园艺. 2025(02): 6-8 .  百度学术
百度学术
2. 姚姚,张浩,王秀云,夏宜平,周泓. SSR分子标记在杜鹃花属植物中的研究进展. 植物遗传资源学报. 2025(03): 405-418 .  百度学术
百度学术
3. 杨松敏,姜潇,程梦雅,王顺顺,段燕如,陈和明,彭东辉. 火焰兰属间杂交育种研究进展. 中国农学通报. 2024(13): 76-82 .  百度学术
百度学术
4. 汪阳,闫三博,张睿,黄琳凯,张新全,聂刚. 白三叶杂交F1代群体的表型鉴定及SSR分析. 草地学报. 2024(06): 1657-1664 .  百度学术
百度学术
5. 夏朝水,曹奕鸯,陈玮婷,甘玮欣,林发壮,许克正,林辉锋. 170份非洲菊种质资源遗传多样性及亲缘关系分析. 福建农业学报. 2024(10): 1120-1129 .  本站查看
本站查看
其他类型引用(0)




 下载:
下载: