Cloning and Prokaryotic Expressions of Cassava MebZIP27
-
摘要:目的
碱性亮氨酸拉链(basic leucine zipper, bZIP)蛋白是真核生物中一类独特的转录因子,在植物的生长、发育和生理代谢调控中发挥着关键作用。特别是A类bZIPs通常负责调控携带ABA依赖元件的基因,进而引发ABA响应或实现环境适应。深入研究A类木薯bZIPs转录因子的功能,可进一步了解其调控ABA表达的分子机理。
方法通过RT-PCR技术克隆MebZIP27基因,并运用生物信息学方法对其进行基因和蛋白质水平的分析。此外,通过荧光定量PCR检测MebZIP27基因的时空表达模式及其对逆境的响应,并尝试通过原核表达获得MebZIP27蛋白。
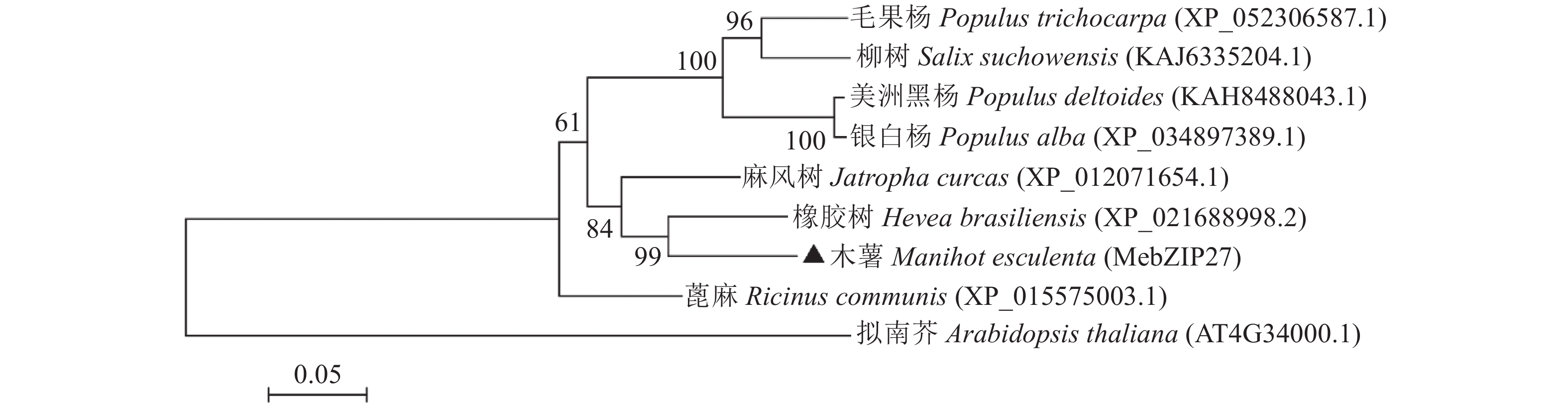
结果MebZIP27基因位于木薯第5号染色体上,编码区全长
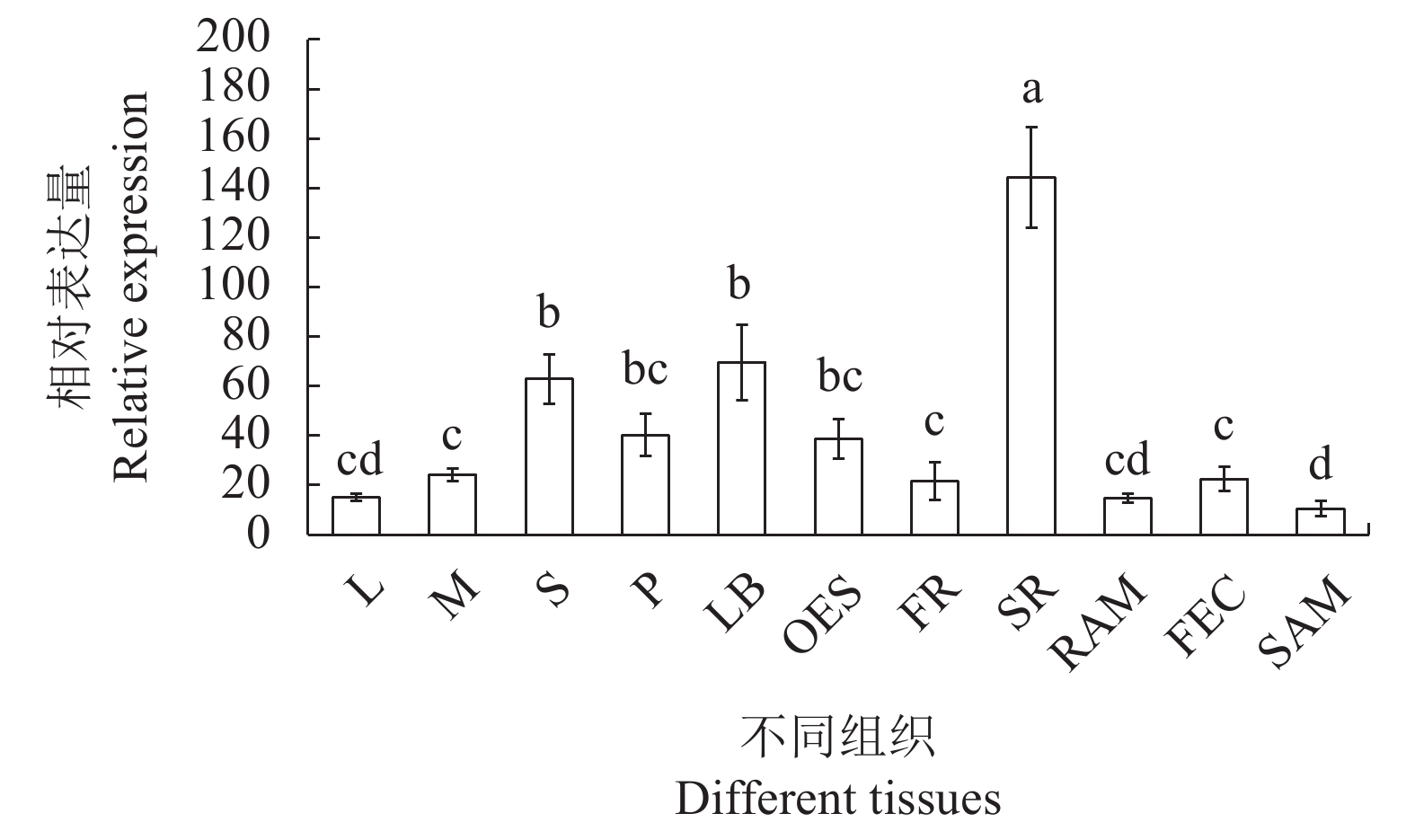
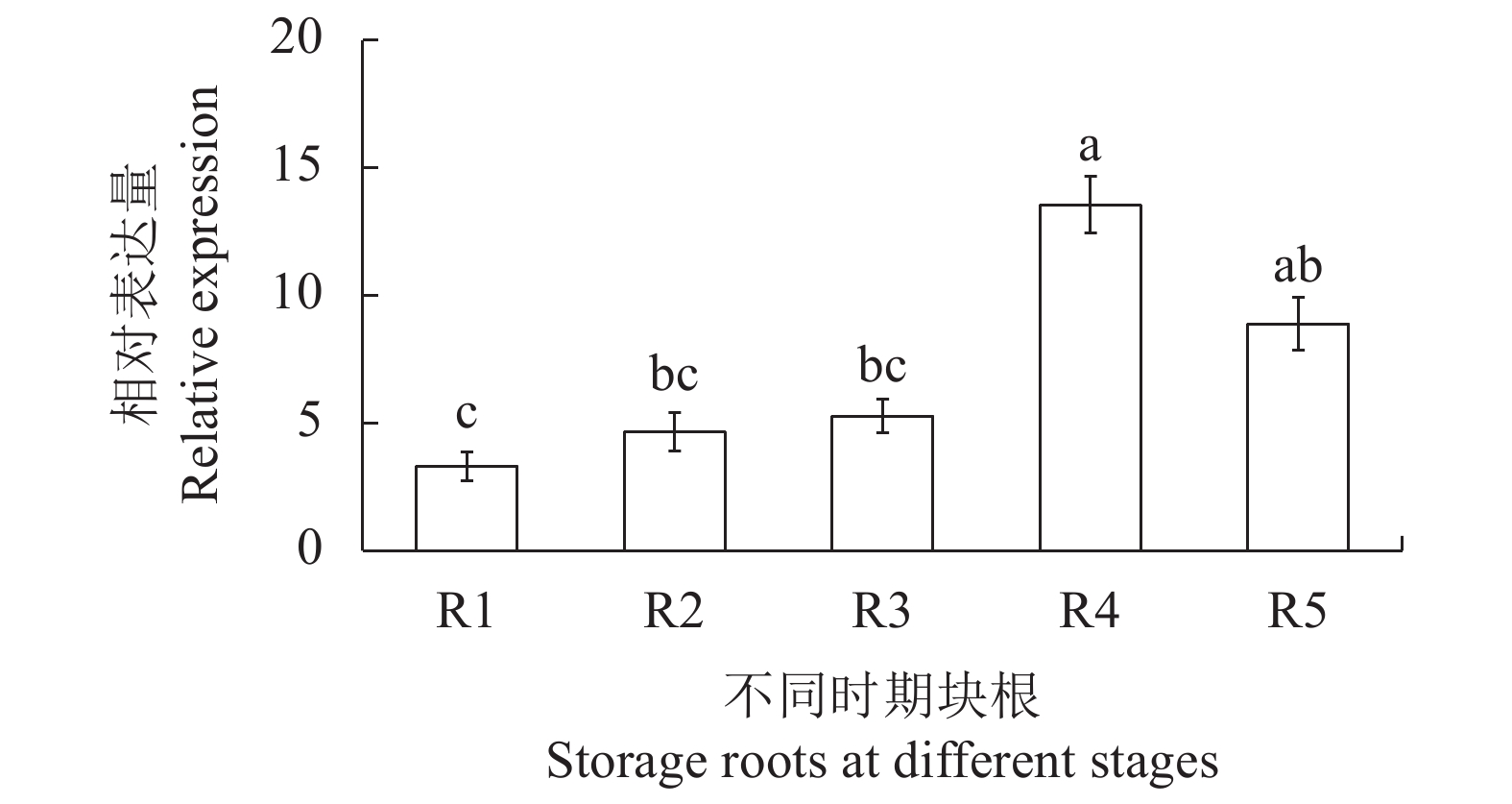
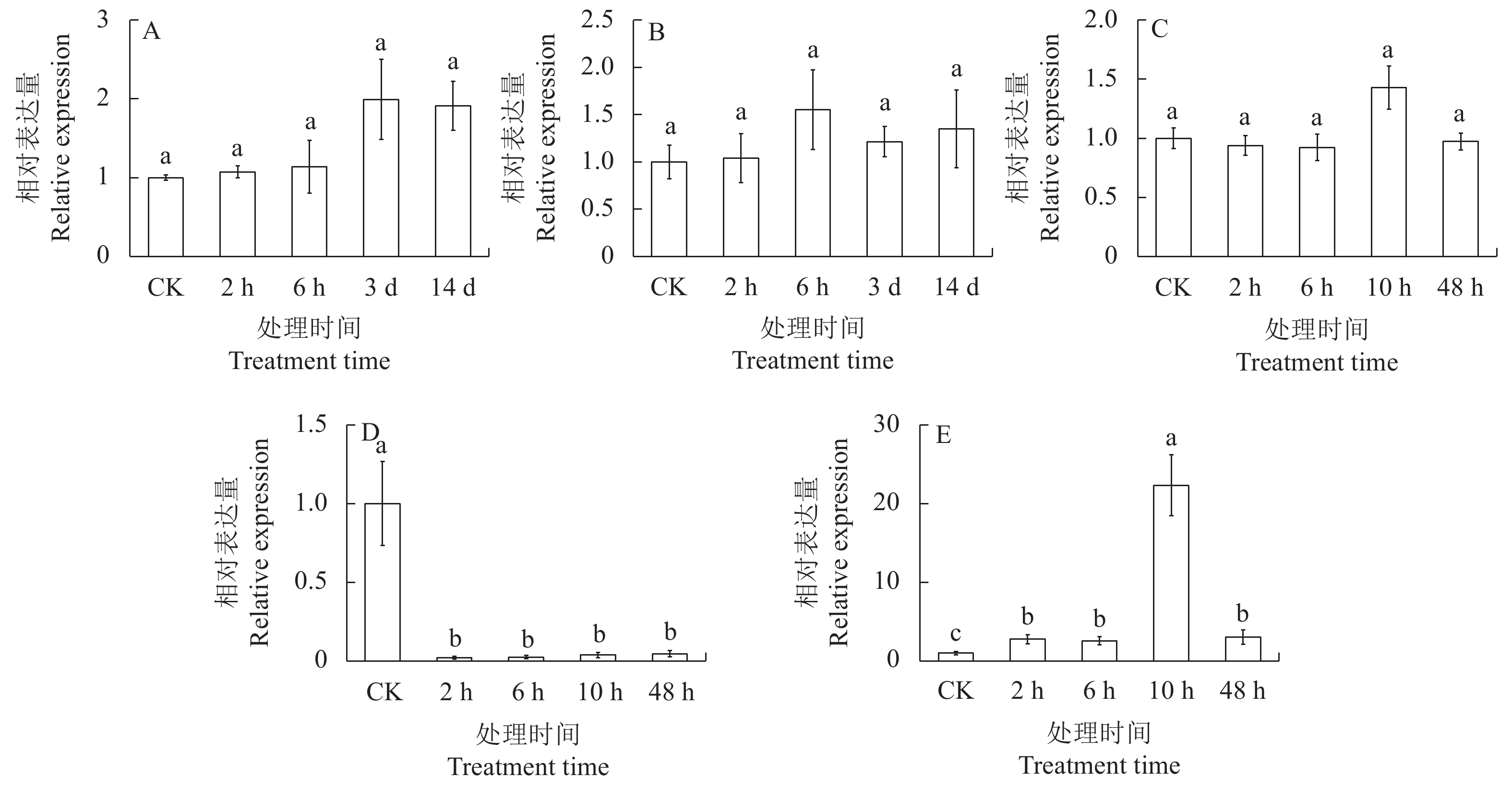
1251 bp,具备典型的bZIP结构域特征。该基因与麻风树(Hevea brasiliensis)和橡胶树(Jatropha curcas)的A类bZIP序列一致性分别高达80.00%和85.41%,表明其属于A类bZIP亚族成员。亚细胞定位结果显示,MebZIP27基因位于细胞核。qRT-PCR分析结果表明,MebZIP27基因在木薯储藏根中表达最为显著。在甘露醇、NaCl和低温处理下,MebZIP27基因未表现出显著诱导表达,但在ABA和H2O2处理下表现出显著诱导。通过原核表达成功获得了MebZIP27基因编码的蛋白,并通过谷胱甘肽琼脂糖珠微球分离技术对GST-MebZIP27蛋白进行了纯化。结论木薯中的MebZIP27基因属于A类bZIP基因家族,定位于细胞核,在木薯储藏根中的表达最为显著。此外,MebZIP27基因在ABA和H2O2处理下表现出显著诱导。这些结果为深入研究MebZIP27基因在ABA信号调控中的作用提供了参考依据。
Abstract:ObjectiveThe Class A basic leucine zipper protein(bZIP), which regulates genes carrying ABA-responsive elements associated with the environmental adaptations as well as growth, development, and physiological metabolism of plants, in cassava was studied.
MethodsThe Class A bZIP in cassava, MebZIP27, was cloned using RT-PCR technology to determine the functions of the gene transcription factors. Bioinformatic methods were applied to analyze properties of the gene and protein, fluorescence quantitative PCR employed to examine spatiotemporal expressions and responses to stress, and prokaryotic expression used to produce MebZIP27 protein.
ResultsMebZIP27 was in the 5th chromosome of cassava with a coding region of
1251 bp and characteristic bZIP domain features. It had the highest homology with the Class A bZIP sequences from Hevea rubber at 80.00% and Jatropha curcas at 85.41%, as a member of the A-bZIP subfamily. Located in nucleus, the gene had the highest expression found in the roots. Treatments of mannitol, NaCl or low temperature did not cause significant induction, but ABA and H2O2 did on it. Via the prokaryotic expression, MebZIP27 could be produced and purified using glutathione agarose bead separation techniques.ConclusionLocated in nucleus and belonged to the Class A bZIP gene family, MebZIP27 expressed most significantly in the storage roots of a cassava plant and by an ABA or H2O2 induction.
-
Keywords:
- cassava /
- bZIP /
- abiotic stress /
- prokaryotic expression
-
微重力影响生命活动的过程和机理,是人类为实现征服太空的目标所必须研究阐明的问题[1]。任何空间微重力试验都需要大量的地面模拟准备试验作为基础,Krikorian等[2]研究发现微重力和模拟微重力条件下胡萝卜细胞发育的不同阶段胚的生长比例相同,说明模拟微重力可以替代太空微重力研究植物生长的试验。地面模拟回转器因经济、易操控,可反复试验,可弥补空间微重力试验条件相对不足并且造价昂贵的缺憾[3-4]。诸多的真实以及模拟微重力对不同植物种类,不同植物部位影响的相关研究表明,微重力对生物的遗传、生长及生理特征等有影响[5],如模拟微重力环境对人参、甜菊、燕麦、向日葵、萝卜等植物生长发育影响的相关报道[6-10]。目前,尚未有模拟微重力对食用菌生长与营养品质影响的相关报道。为此,本研究利用三维回转器模拟微重力环境,探讨微重力作用对金针菇氨基酸营养成分的影响,为揭示微重力对食用菌生长发育机理及开展空间园艺与育种提供科学借鉴。
1. 材料与方法
1.1 供试菌种
试验于2015年开展研究,金针菇品种由福建省农业科学院土壤肥料研究所提供。
1.2 回转装置
三维回转装置为福建省农业科学院生态农业研究所在国家“863”计划支持下自主研发的适合植物湿润栽培的旋转式植物栽培装置(中国发明专利:ZL200710009491.6)。装置设置两套独立旋转机构,三维旋转栽培盘在绕着自转心轴旋转的同时,又绕着公转心轴旋转。机架内部设置受控密闭舱,三维旋转栽培盘置于舱内,舱内气体组分、调速电机转速、人工光源光照强度和光周期等技术参数也可根据试验要求进行调控,采用触摸屏作为人机交互界面[11-12]。
1.3 试验方法与设计
1.3.1 栽培设计
试验设2个处理,分别为模拟微重力金针菇处理(X3) 与静止栽培金针菇处理(X0)。
金针菇培养料配方:棉籽壳77%,石膏1%,白糖1%,石灰2%,玉米粉19%,pH自然。培养料料水比1:1.8,拌匀后装入塑料袋,每袋装干料230 g,套上封口环,环内塞棉花,高压灭菌。待培养料温度冷却至26℃左右接种,每处理3个重复,每个重复10袋。接种后的菌袋置于回转装置上避光培养,培养温度22~23℃,空气相对湿度70%~75%。出菇时环境温度控制在18~21℃,空气相对湿度控制在90%~93%。三维回转装置频率1/60。
1.3.2 氨基酸组成测定
采收子实体菌盖呈钟型,尚未开伞时的金针菇。烘干箱中75℃烘干,粉碎机中磨粉150目过筛,用密封袋包装后在干燥环境中保藏备用。
将烘干样品置于6 mol·L-1盐酸溶液中,于110℃水解24 h,用氨基酸自动分析仪(日立8801型)测定氨基酸含量[13]。
1.4 数据统计与分析
氨基酸评分(Amino Acid Score,AAS)、生物价(Biologica1 Value,BV)、必需氨基酸指数(Essential Amino Acid Index,EAAI)和营养指数(Nutritional Index,NI)采用Bano的方法[14];化学评分(chemical score,CS)采用FAO确定的方法[15];氨基酸比值系数(RCAA)与氨基酸比值系数分(SRCAA)按朱圣陶的方法测定[16]。
2. 结果与分析
2.1 模拟微重力和静止栽培的金针菇氨基酸含量
X3与X0处理的金针菇子实体中所含17种氨基酸种类完全相同,模拟微重力栽培金针菇子实体中有15种氨基酸含量高于静止栽培处理,氨基酸总量达151.2 g·kg-1,比静止栽培处理提高了26.3%。模拟微重力处理的金针菇必需氨基酸总量81.7 g·kg-1,比静止栽培处理提高了29.89%,其必需氨基酸总量与氨基酸总量的比值为54.03%,超过食物类的必需氨基酸与氨基酸总含量的比值应接近40%的标准[17]。天冬氨酸和谷氨酸是鲜味氨基酸,模拟微重力处理的鲜味氨基酸含量达34.7 g·kg-1,比静止栽培处理的提高了37.15%。说明模拟微重力效应不仅可以提高金针菇的各种类氨基酸的含量,尤其对提高必需氨基酸和鲜味氨基酸含量有显著的增效效应(表 1)。
表 1 模拟微重力与静止栽培的金针菇子实体氨基酸含量比较Table 1. AA in fruiting bodies of F. velutipescultivated under simulated microgravity and conventional method[单位/(g·kg-1)] 氨基酸 X0 X3 增幅/% 天门冬氨酸Asp 9.9 15.5 56.6 苏氨酸Thr 8.9 10.2 14.6 丝氨酸Ser 5.7 7.6 33.3 谷氨酸Glu 15.4 19.2 24.7 甘氨酸Gly 5.4 6.6 22.2 丙氨酸Ala 7.2 8.5 18.1 半胱氨酸Cys 2.4 3.5 45.8 缬草氨酸Val 6.0 8.3 38.3 甲硫氨酸Met 9.6 10.3 7.3 异亮氨酸Ile 6.3 7.7 22.2 亮氨酸Leu 7.9 11.7 48.1 酪氨酸Tyr 4.2 5.5 31.0 苯丙氨酸Phe 5.6 8.3 48.2 赖氨酸Lys 12.0 16.2 35.0 组氨酸His 2.7 2.8 3.7 精氨酸Arg 5.8 4.7 -19.0 脯氨酸Pro 4.7 4.6 -2.1 氨基酸总量 119.7 151.2 26.30 必需氨基酸总量 62.9 81.7 29.89 鲜味氨基酸总量 25.3 34.7 37.15 2.2 模拟微重力和静止栽培的金针菇必需氨基酸组成及含量
必需氨基酸含量(Essential Amino Acids,EAA)指必需氨基酸含量分别占总氨基酸含量的比例[18]。模拟微重力环境栽培的金针菇必需氨基酸含量比静止栽培处理、鸡蛋白、FAO/WHO的参照标准分别提高了2.82%、8.71%和52.63%(表 2)。此外,模拟微重力处理的缬草氨酸、亮氨酸、苯丙氨酸+酪氨酸和赖氨酸含量比静止栽培处理的子实体提高l1.34%、17.27%、11.48%和6.78%,而苏氨酸、蛋氨酸+胱氨酸、异亮氨酸则低了14.67%、9.86%和3.34%。参照世界粮农和卫生组织标准和鸡蛋白的氨基酸营养模式,从表 2还发现,模拟微重力和静止栽培的金针菇蛋白质必需氨基酸的含量均高于世界粮农和卫生组织规定的标准和鸡蛋白的含量。
表 2 模拟微重力和静止栽培的金针菇子实体必需氨基酸组成及含量Table 2. Compositions and contents of essential AA in fruiting bodies of F. velutipes cultivated under simulated microgravity and conventional method处理 苏氨酸
Thr蛋氨酸+胱氨酸
Met+Cys缬草氨酸
Val异亮氨酸
Ile亮氨酸
Leu苯丙氨酸+酪氨酸Phe+Tyr 赖氨酸
Lys总量 X0 7.44 10.03 5.01 5.26 6.60 8.19 10.03 52.55 X3 6.75 9.13 5.49 5.09 7.74 9.13 10.71 54.03 鸡蛋白 5.1 5.5 7.3 6.6 8.8 10.0 6.4 49.7 FAO/WHO 4.0 3.5 5.0 4.4 7.0 6.0 5.5 35.4 2.3 模拟微重力和静止栽培的金针菇蛋白质化学评分
模拟微重力栽培金针菇子实体的化学评分比静止栽培处理高6.63%,t测验的差异达到显著水平。缬草氨酸、亮氨酸和苯丙氨酸+酪氨酸和赖氨酸评分值比静止栽培处理提高6.63%、14.10%、8.40%和3.91%,而苏氨酸、蛋氨酸+胱氨酸、异亮氨酸则低了13.31%、12.98%和6.20%(表 3)。
表 3 模拟微重力和静止栽培的金针菇的蛋白质化学评分Table 3. CS of proteins in fruiting bodies of F. velutipes cultivated under simulated microgravity and conventional method处理 苏氨酸
Thr蛋氨酸+胱氨酸
Met+Cys缬草氨酸
Val异亮氨酸
Ile亮氨酸
Leu苯丙氨酸+酪氨酸Phe+Tyr 赖氨酸
Lys化学评价 X0 137.9 172.4 64.9 75.4 70.9 77.4 148.2 64.9 X3 121.7 152.6 69.2 71.0 80.9 83.9 154.0 69.2 2.4 模拟微重力和静止栽培的金针菇氨基酸评分
模拟微重力栽培金针菇子实体的氨基酸评分比静止栽培处理高16.43%,t测验的差异达到显著水平。缬草氨酸、亮氨酸和苯丙氨酸+酪氨酸和赖氨酸评分比静止栽培高9.47%、17.18%、11.43%和6.86%,而苏氨酸、蛋氨酸+胱氨酸、异亮氨酸则低了10.20%、9.82%和3.37%。
2.5 模拟微重力和静止栽培的金针菇必需氨基酸指数、生物价和营养指数
模拟微重力栽培金针菇子实体的必需氨基酸指数、生物价、营养指数均高于静止栽培处理的相对应值,其依次比静止栽培处理高2.92%、3.21%和29.37%,这表明模拟微重力栽培金针菇子实体的各类氨基酸组分不仅含量高于静止栽培处理,相应的氨基酸组成比例较为合理。
表 4 模拟微重力和静止栽培金针菇的氨基酸评分Table 4. AAS of proteins in fruiting bodies of F. velutipes cultivated under simulated microgravity and conventional method处理 苏氨酸
Thr蛋氨酸+胱氨酸
Met+Cys缬草氨酸
Val异亮氨酸
Ile亮氨酸
Leu苯丙氨酸+酪氨酸Phe+Tyr 赖氨酸
Lys氨基酸评分 X0 185.9 286.4 100.3 119.6 94.3 136.5 182.3 94.3 X3 168.7 260.8 109.8 115.7 110.5 152.1 194.8 109.8 表 5 模拟微重力和静止栽培金针菇的必需氨基酸指数、生物价和营养指数Table 5. EAAI, BVand NI of proteins in fruitingbodies of F. velutipes cultivated under simulated microgravity and conventional method处理 必需氨基酸指数 生物价 营养指数 X0 104.92 102.7 12.6 X3 107.98 106.0 16.3 2.6 模拟微重力和静止栽培对金针菇氨基酸比值和比值系数分
根据WHO/FAO的必需氨基酸评分模式,RC值>1表明该氨基酸相对过剩,RC值<1则表明不足,RC最低者为第一限制性氨基酸。如果必需氨基酸组成含量组成与EAA模式一致,则SRC=100,与EAA模式越接近,则SRC越接近100,其营养价值越高[18]。模拟微重力栽培金针菇子实体的氨基酸比值系数分(SRCAA)高于静止栽培处理,其相应值比静止栽培处理高0.66%。根据必需氨基酸模式,静止栽培的金针菇必需氨基酸——亮氨酸的氨基酸比值系数为0.9,小于WHO/FAO模式的必需氨基酸的RC值1,说明静止栽培的金针菇的第一限制氨基酸是亮氨酸。但是模拟微重力栽培的金针菇中亮氨酸的氨基酸比值系数为1.2,高于WHO/FAO模式的RC值,而且其他必需氨基酸的氨基酸比值系数均高于1,表明模拟微重力栽培金针菇的蛋白质是优质蛋白质。
表 6 模拟微重力和静止栽培金针菇的氨基酸比值系数分Table 6. AARC of proteins in fruiting bodies of F. velutipes cultivated under simulated microgravity and conventional method处理 苏氨酸
Thr蛋氨酸+胱氨酸
Met+Cys缬草氨酸
Val异亮氨酸
Ile亮氨酸
Leu苯丙氨酸+酪氨酸Phe+Tyr 赖氨酸
Lys氨基酸比值系数分 X0 1.86 2.86 1.00 1.20 0.94 1.36 1.82 72.68 X3 1.69 2.61 1.10 1.16 1.11 1.52 1.95 73.16 2.7 模拟微重力和静止栽培的金针菇蛋白质营养价值影响的综合评价
从表 7看出,模拟微重力栽培金针菇的化学评分、氨基酸评分、氨基酸比值系数分、必需氨基酸指数、营养指数及生物价6项蛋白质指标均高于静止栽培的金针菇,说明模拟微重力有利于金针菇子实体的氨基酸合成与积累,不仅氨基酸总量高,而且各种氨基酸组成比例更为合理。根据通用的蛋白质营养价值评判标准,模拟微重力栽培金针菇子实体中蛋白质综合营养价值优于静止栽培处理。
表 7 模拟微重力和静止栽培金针菇的蛋白质营养综合评价Table 7. Over-allnutritional qualities of proteins in fruiting bodies of F. velutipes cultivated under simulated microgravity and conventional method处理 化学评分 氨基酸评分 氨基酸比值系数分 必需氨基酸指数 营养指数 生物价 X0 64.9 94.3 72.68 104.92 12.6 102.7 X3 69.2 109.8 73.16 107.98 16.3 106.0 3. 讨论与结论
诸多研究发现,微重力对作物的生长、发育和繁殖产生一定的影响,而且多是以不利影响为主。Giuseppe等[19]研究空间实验微重力对芸芥发芽率、苗干鲜重、葡萄糖与果糖含量、蔗糖和淀粉含量有不利影响;徐国鑫等[20]发现模拟微重力抑制拟南芥种子贮藏蛋白的积累,导致种子贮藏蛋白总体含量降低。本研究模拟微重力栽培的金针菇子实体17种氨基酸中有15种氨基酸含量高于静止栽培处理,且氨基酸、必需氨基酸和鲜味氨基酸总量均高于静止处理,说明模拟微重力栽培有利于金针菇中蛋白质物质的代谢与积累,其结果与赵伟等[21]研究经回转器处理的人参细胞的人参皂苷含量提高10%左右相似。为此模拟微重力的相关试验要因作物而异,在今后的科学研究中需根据具体作物具体分析。
由于食物蛋白质中一种或几种必需氨基酸缺少或不足,就会使食物蛋白质合成为机体蛋白质的转变过程受限,进而限制了此种蛋白质的营养价值[22]。本研究的静止栽培金针菇中亮氨酸的氨基酸评分与必需氨基酸比值系数均最小,因此亮氨酸是限制氨基酸;而模拟微重力栽培的金针菇限制氨基酸是缬草氨酸,苯丙氨酸+酪氨酸的必需氨基酸比值系数RC值大于1。因此模拟微重力栽培可提升秀珍菇中氨基酸的含量,尤其是限制性氨基酸——苯丙氨酸+酪氨酸的含量,从而更为接近人体蛋白质各种氨基酸的构成比例,易于更完全被人体吸收转化。
模拟微重力栽培金针菇子实体中有15种氨基酸含量高于静止栽培处理,而且化学评分、氨基酸评分、氨基酸比值系数分、必需氨基酸指数、生物价与营养指数6项蛋白质指标均高于静止栽培的金针菇,说明模拟微重力栽培金针菇有利于其氨基酸的形成,不仅能提高蛋白质含量,而且促进了各种氨基酸构成比例的合理性,使之更为接近人体蛋白质各种氨基酸的构成比例,其作用机理有待于进一步的探索。微重力是否对维生素、脂肪酸、多糖、微量元素以及重金属的作用也有待于深入研究。
-
图 1 MebZIP27基因的克隆
M:DNA标准物;1:基因克隆产物样品,2~7:菌液PCR样品;A:ABA处理下的表达分析(数值为相比0 h的表达量并对值取log2);*和**分别表示与0 h相比在0.05和0.01水平上差异显著。B:MebZIP27基因克隆;C:MebZIP27基因片段的菌液PCR检测;D:结构域分析。
Figure 1. Cloning of MebZIP27
M: DNA marker; 1: sample of gene cloning product; 2–7: samples of bacterial broth. A: MebZIP27 expression under ABA treatment (log2 value relative to 0 h); * and **: significant differences at 0.05 and 0.01 levels, respectively. B: clone of MebZIP27. C: PCR detection of MebZIP27 segment in bacterial broth. D: domain analysis.
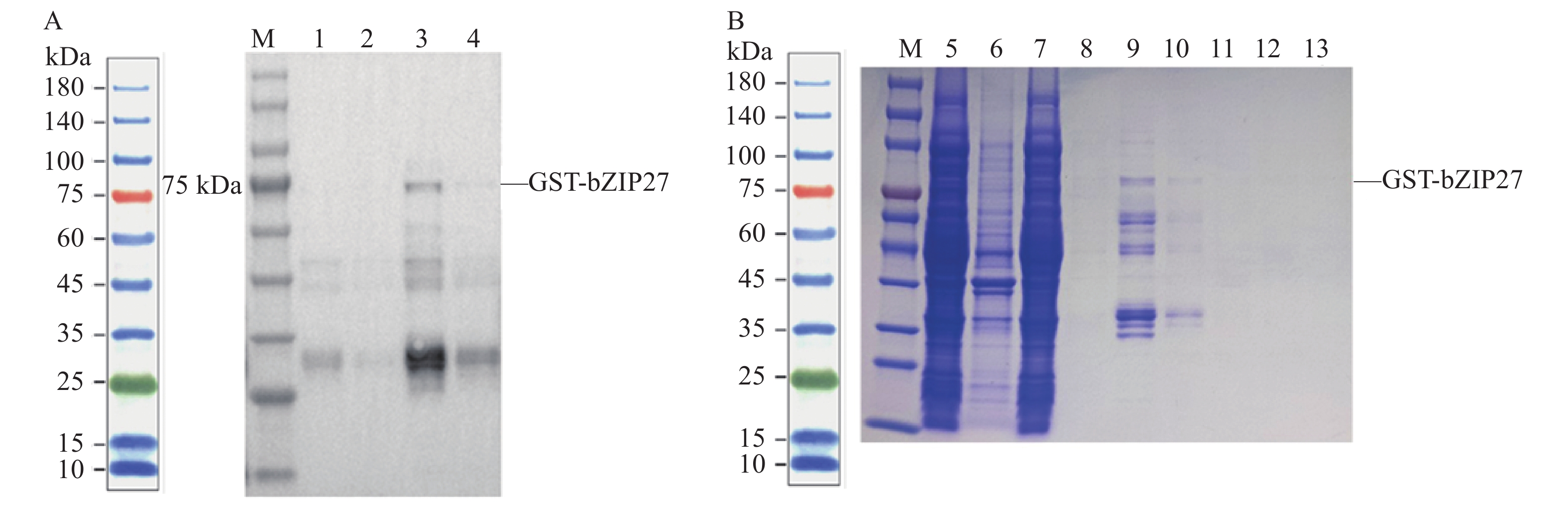
图 8 MebZIP27蛋白的原核表达
M:蛋白标准物;1:18 ℃ 0.2 mmol·L−1 IPTG诱导过夜上清;2:18 ℃ 0.2 mmol·L−1 IPTG诱导过夜沉淀;3:37 ℃ 0.2 mmol·L−1 IPTG诱导过夜上清;4:37 ℃ 0.2 mmol·L−1 IPTG诱导过夜沉淀;5:细胞裂解后上清;6:细胞裂解后沉淀;7:流穿液;8:20 mmol·L−1GSH洗脱液1;9:20 mmol·L−1GSH洗脱液2;10:20 mmol·L−1GSH洗脱液3;11:20 mmol·L−1GSH洗脱液4;12:20 mmol·L−1GSH洗脱液5;13:20 mmol·L−1GSH洗脱液6。
Figure 8. Prokaryotic expression of MebZIP27 protein
M: protein ladder; 1: supernatant after overnight induction in 0.2 mmol·L−1 IPTG at 18 ℃; 2: precipitate after overnight induction in 0.2 mmol·L−1 IPTG at 18 ℃; 3: supernatant after overnight induction in 0.2 mmol·L−1 IPTG at 37 ℃; 4: precipitate after overnight induction in 0.2 mmol·L−1 IPTG at 37 ℃; 5: supernatant after cell lysis; 6: pellet after cell lysis; 7: flow-through; 8: elution buffer 1 with 20 mmol·L−1 GSH; 9: elution buffer 2 with 20 mmol·L−1 GSH; 10: elution buffer 3 with 20 mmol·L−1 GSH; 11: elution buffer 4 with 20 mmol·L−1 GSH; 12: elution buffer 5 with 20 mmol·L−1 GSH; 13: elution buffer 6 with 20 mmol·L−1 GSH.
表 1 引物序列
Table 1 Primers applied
引物名称
Primer上游序列
Forward primers (5'-3')下游序列
Reverse primers (5'-3')用途
UsageMebZIP27 ATGGGGACTCATCTCAACTTCAAGAACTTC TTACCATGGACCAGTCTGAGTCCTC 基因克隆 MebZIP27Q GAGCAGCAGGGAGTTA TACGCAAAGTGAGACG 实时荧光定量PCR TUB TGCCATGTTCCGTGGAAAGATG CCCCTAGGTGGAATGTCACAGACAC 实时荧光定量PCR MebZIP27-P TTCCAGGGGCCCCTGGGATCATGGGGACTCATCTC
AACTTCAAGAACTTCGTCACGATGCGGCCGCTCGATTACCATGGACCAGT
CTGAGTCCTC原核表达 -
[1] 崔荣秀,张议文,陈晓倩,等. 植物bZIP参与胁迫应答调控的最新研究进展[J]. 生物技术通报,2019,35(2) :143−155. CUI R X,ZHANG Y W,CHEN X Q,et al. The latest research progress on the stress responses of bZIP involved in plants[J]. Biotechnology Bulletin,2019,35(2) :143−155. (in Chinese)
[2] HAN H,WANG C N,YANG X Y,et al. Role of bZIP transcription factors in the regulation of plant secondary metabolism[J]. Planta,2023,258(1) :13. DOI: 10.1007/s00425-023-04174-4
[3] WEI K,CHEN J,WANG Y,et al. Genome-wide analysis of bZIP-encoding genes in maize[J]. DNA Research,2012,19(6) :463−476. DOI: 10.1093/dnares/dss026
[4] LIU X,CHU Z Q. Genome-wide evolutionary characterization and analysis of bZIP transcription factors and their expression profiles in response to multiple abiotic stresses in Brachypodium distachyon[J]. BMC Genomics,2015,16(1) :227. DOI: 10.1186/s12864-015-1457-9
[5] JAKOBY M,WEISSHAAR B,DRÖGE-LASER W,et al. bZIP transcription factors in Arabidopsis[J]. Trends in Plant Science,2002,7(3) :106−111. DOI: 10.1016/S1360-1385(01)02223-3
[6] KANG C,ZHAI H,HE S Z,et al. A novel sweetpotato bZIP transcription factor gene,IbbZIP1,is involved in salt and drought tolerance in transgenic Arabidopsis[J]. Plant Cell Reports,2019,38(11) :1373−1382. DOI: 10.1007/s00299-019-02441-x
[7] LIU H T,TANG X,ZHANG N,et al. Role of bZIP transcription factors in plant salt stress[J]. International Journal of Molecular Sciences,2023,24(9) :7893. DOI: 10.3390/ijms24097893
[8] 赵婉莹,于太飞,杨军峰,等. 大豆GmbZIP16的抗旱功能验证及分析[J]. 中国农业科学,2018,51(15) :6−18. DOI: 10.3864/j.issn.0578-1752.2018.15.001 ZHAO W Y,YU T F,YANG J F,et al. Verification and analyses of soybean GmbZIP16 gene resistance to drought[J]. Scientia Agricultura Sinica,2018,51(15) :6−18. (in Chinese) DOI: 10.3864/j.issn.0578-1752.2018.15.001
[9] UMEZAWA T,NAKASHIMA K,MIYAKAWA T,et al. Molecular basis of the core regulatory network in ABA responses:Sensing,signaling and transport[J]. Plant & Cell Physiology,2010,51(11) :1821−1839.
[10] TAO R,LIU Y Q,CHEN S,et al. Meta-analysis of the effects of overexpressed bZIP transcription factors in plants under drought stress[J]. Plants,2024,13(3) :337. DOI: 10.3390/plants13030337
[11] NAKASHIMA K,YAMAGUCHI-SHINOZAKI K,SHINOZAKI K. The transcriptional regulatory network in the drought response and its crosstalk in abiotic stress responses including drought,cold,and heat[J]. Frontiers in Plant Science,2014,5:170.
[12] FUJITA Y,FUJITA M,SATOH R,et al. AREB1 is a transcription activator of novel ABRE-dependent ABA signaling that enhances drought stress tolerance in Arabidopsis[J]. The Plant Cell,2005,17(12) :3470−3488. DOI: 10.1105/tpc.105.035659
[13] JULKOWSKA M M,TESTERINK C. Tuning plant signaling and growth to survive salt[J]. Trends in Plant Science,2015,20(9) :586−594. DOI: 10.1016/j.tplants.2015.06.008
[14] TODAKA D,SHINOZAKI K,YAMAGUCHI-SHINOZAKI K. Recent advances in the dissection of drought-stress regulatory networks and strategies for development of drought-tolerant transgenic rice plants[J]. Frontiers in Plant Science,2015,6:84.
[15] HU W,JI C M,SHI H T,et al. Allele-defined genome reveals biallelic differentiation during cassava evolution[J]. Molecular Plant,2021,14(6) :851−854. DOI: 10.1016/j.molp.2021.04.009
[16] 曾坚,吴伟姗,谢彩虹,等. 木薯MeERF1.2基因克隆及表达分析[J]. 福建农业学报,2023,38(4) :410−416. ZENG J,WU W S,XIE C H,et al. Cloning and expression of MeERF1.2 in cassava[J]. Fujian Journal of Agricultural Sciences,2023,38(4) :410−416. (in Chinese)
[17] HU W,WANG L Z,TIE W W,et al. Genome-wide analyses of the bZIP family reveal their involvement in the development,ripening and abiotic stress response in banana[J]. Scientific Reports,2016,6:30203. DOI: 10.1038/srep30203
[18] LI X L,FAN S H,HU W,et al. Two cassava basic leucine zipper(bZIP) transcription factors(MebZIP3 and MebZIP5) confer disease resistance against cassava bacterial blight[J]. Frontiers in Plant Science,2017,8:2110. DOI: 10.3389/fpls.2017.02110
[19] 颜彦,丁泽红,铁韦韦,等. 木薯MeSnRK2-1基因克隆及表达分析[J]. 分子植物育种,2018,16(15) :4839−4844. YAN Y,DING Z H,TIE W W,et al. Cloning and expression analysis of MeSnRK2-1 gene in cassava[J]. Molecular Plant Breeding,2018,16(15) :4839−4844. (in Chinese)
[20] WILSON M C,MUTKA A M,HUMMEL A W,et al. Gene expression atlas for the food security crop cassava[J]. New Phytologist,2017,213(4) :1632−1641. DOI: 10.1111/nph.14443
[21] ZHANG Y,ZHANG G,XIA N,et al. Cloning and characterization of a bZIP transcription factor gene in wheat and its expression in response to stripe rust pathogen infection and abiotic stresses[J]. Physiological and Molecular Plant Pathology,2008,73(4/5) :88−94.
[22] MIAO Z H,LIU X,LAM E. TGA3 is a distinct member of the TGA family of bZIP transcription factors in Arabidopsis thaliana[J]. Plant Molecular Biology,1994,25(1) :1−11. DOI: 10.1007/BF00024193
[23] LEE B J,PARK C J,KIM S K,et al. In vivo binding of hot pepper bZIP transcription factor CabZIP1 to the G-box region of pathogenesis-related protein 1 promoter[J]. Biochemical and Biophysical Research Communications,2006,344(1) :55−62. DOI: 10.1016/j.bbrc.2006.03.153
[24] ZHOU J W,DU G C,CHEN J. Novel fermentation processes for manufacturing plant natural products[J]. Current Opinion in Biotechnology,2014,25:17−23. DOI: 10.1016/j.copbio.2013.08.009
[25] KIM S,KANG J Y,CHO D I,et al. ABF2,an ABRE-binding bZIP factor,is an essential component of glucose signaling and its overexpression affects multiple stress tolerance[J]. The Plant Journal,2004,40(1) :75−87. DOI: 10.1111/j.1365-313X.2004.02192.x
[26] LIU C T,MAO B G,OU S J,et al. OsbZIP71,a bZIP transcription factor,confers salinity and drought tolerance in rice[J]. Plant Molecular Biology,2014,84(1/2) :19−36.
[27] HOSSAIN M A,CHO J I,HAN M,et al. The ABRE-binding bZIP transcription factor OsABF2 is a positive regulator of abiotic stress and ABA signaling in rice[J]. Journal of Plant Physiology,2010,167(17) :1512−1520. DOI: 10.1016/j.jplph.2010.05.008
[28] ZG E,ZHANG Y P,ZHOU J H,et al. Mini review roles of the bZIP gene family in rice[J]. Genetics and Molecular Research,2014,13(2) :3025−3036. DOI: 10.4238/2014.April.16.11
[29] AMIR HOSSAIN M,LEE Y,CHO J I,et al. The bZIP transcription factor OsABF1 is an ABA responsive element binding factor that enhances abiotic stress signaling in rice[J]. Plant Molecular Biology,2010,72(4/5) :557−566.
[30] XIANG Y,TANG N,DU H,et al. Characterization of OsbZIP23 as a key player of the basic leucine zipper transcription factor family for conferring abscisic acid sensitivity and salinity and drought tolerance in rice[J]. Plant Physiology,2008,148(4) :1938−1952. DOI: 10.1104/pp.108.128199
[31] ZOU M J,GUAN Y C,REN H B,et al. A bZIP transcription factor,OsABI5,is involved in rice fertility and stress tolerance[J]. Plant Molecular Biology,2008,66(6) :675−683. DOI: 10.1007/s11103-008-9298-4
[32] NICOLAS P,LECOURIEUX D,KAPPEL C,et al. The basic leucine zipper transcription factor abscisic acid response element-binding factor2 is an important transcriptional regulator of abscisic acid-dependent grape berry ripening processes[J]. Plant Physiology,2014,164(1) :365−383. DOI: 10.1104/pp.113.231977
[33] COSTA A,DRAGO I,BEHERA S,et al. H2O2 in plant peroxisomes:An in vivo analysis uncovers a Ca2+-dependent scavenging system[J]. The Plant Journal,2010,62(5) :760−772. DOI: 10.1111/j.1365-313X.2010.04190.x
-
期刊类型引用(3)
1. 王广慧,魏雅冬,于德涵,张腾霄,王斌. 益生菌发酵制备金针菇抗氧化肽的研究. 饲料研究. 2023(04): 95-100 .  百度学术
百度学术
2. 胡忠玲. 生态环境保护对金针菇增产效应的影响分析. 中国食用菌. 2019(09): 116-119 .  百度学术
百度学术
3. 陶永新,段静怡,李依宁,李自燕,宋寒冰,张祺锶,黄嘉华,高玲玲,谢宝贵. 金针菇L-赖氨酸合成通路基因鉴定及对不同光质的响应表达. 食用菌学报. 2018(04): 1-8 .  百度学术
百度学术
其他类型引用(0)




 下载:
下载: