Prodigiosin-producing Genes in Serratia plymuthica ACCC 02146
-
摘要:目的 鉴定影响普城沙雷氏菌ACCC 02146产灵菌红素能力的基因,构建ACCC 02146的转座子突变体文库,为进一步研究普城沙雷氏菌ACCC 02146灵菌红素合成机制奠定基础。方法 采用平板划线法从菌种保藏中心购买菌株和实验室保藏菌株中获得15株产灵菌红素的菌株。采用16S rRNA基因测序法鉴定各菌株,邻接建树将其分类,并比较不同类别菌株灵菌红素合成基因簇启动子序列差异,观察各菌株产色能力。对16S rDNA序列和灵菌红素合成基因簇启动子序列均有异于其他菌株的普城沙雷氏菌ACCC 02146合成灵菌红素的调控基因展开研究。构建ACCC 02146的转座子突变体文库,筛选出产色能力明显改变的克隆子并鉴定出对应的转座子插入突变基因。结果 该突变体文库中有74个突变体表现出产灵菌红素能力的变化。其中25个突变体转座子插入发生在pigA、pigB、pigC、pigD和pigH 等5个灵菌红素合成簇基因上,49个突变体转座子插入基因为灵菌红素合成基因簇之外的基因。在鉴定到的产色能力改变的突变体中,麦芽糖O-乙酰基转移酶基因突变菌株有6个,二氢乳清酸脱氢酶基因突变菌株有4个,MarR家族转录因子SlyA基因突变体有3个,winged helix家族的双组分转录调控因子RstA基因突变体有3个,H-醌氧化还原酶亚基I基因突变菌株有3个,NADH-醌氧化还原酶G链基因突变菌株有3个,肽基脯氨酰异构酶B基因突变菌株有3个,其他突变基因对应的克隆子数量为1~2个。结论 在沙雷氏菌中,除了灵菌红素合成簇基因调控灵菌红素合成,推测灵菌红素合成簇外编码相关酶、转录调控因子和一些结构蛋白的基因通过直接或间接途径在不同程度上调控了灵菌红素的合成。Abstract:Objective Genes relating to prodigiosin synthesis in Serratia plymuthica ACCC 02146 were identified, and a transposon mutant library on the strain constructed.Methods Fifteen prodigiosin-producing microbes were obtained from a conservation center and a laboratory. After identification by 16S rRNA gene sequencing, they were classified according to the neighbor-joining trees. Promoter sequences of prodigiosin synthesis gene were analyzed, and color producing capacity of the individual strains evaluated. Selected strain was cloned and further studied to establish a library on the transposon mutants.Results Differed from other strains in terms of 16S rDNA and promoter sequences of the prodigiosin biosynthesis gene clusters, S.plymuthica ACCC 02146 was selected to clone the candidate gene for further investigation. A transposon mutant library was constructed subsequently. In the library, of 74 mutants showing significant variations in prodigiosin-producing ability, 25 had the insertions in pigA, pigB, pigC, pigD, and pigH, while 49 in the genes outside the cluster. On color formation, 6 strains with the mutation on maltose o-acetyltransferase gene, 4 on dihydroorotate dehydrogenase gene, 3 on MarR family of transcription factor SlyA genes, 3 on two-component transcriptional regulator RstA of the winged helix family, 3 on NAD(P)H-quinone oxidoreductase subunit I gene, 3 on NADH-quinone oxidoreductase, chain G gene, 3 on peptidylprolyl isomerase B gene, and one to two on other genes were found possibly related to significant alterations on the prodigiosin production as well.Conclusion Aside from the identified specific clusters of prodigiosin synthesis-associated genes, additional factors in the forms of enzymes, transcriptional regulators, and/or structural proteins were now speculated to also directly or indirectly contribute in varying degrees to the prodigiosin synthesis in Serratia sp.
-
Keywords:
- Serratia marcescens /
- prodigiosin /
- transposon /
- mutant library /
- regulatory gene
-
0. 引言
【研究意义】绵羊肺炎支原体(Mycoplasma ovipneumoniae, Mo)是引起绵羊和山羊支原体性肺炎(Mycoplasma pneumonia of goats and sheep, MPGS)的一种经呼吸道传染的病原[1]。MPGS患病羊主要表现为肺炎、咳嗽、流鼻涕、体温升高、摄食量下降、腹泻便秘、精神状态恶化和生长发育迟缓等症状[2-3]。该病一年四季都会发生,冬春季节多发,发病率30%~50%[4-7],主要在甘肃、安徽、山东、四川、贵州、福建等地流行[8]。山羊伪结核棒状杆菌(Corynebacterium pseudotuberculosis, CP)是山羊伪结核病的病原,山羊伪结核病是一种人畜共患的接触性传染病[9],也称为干酪样淋巴结炎(Caseous lymphadenitis, CLA)。患病羊主要表现为消瘦、体温升高、食欲减退、生产性能降低,孕畜出现流产、死胎、畸胎等现象[10]。其发病率一般在8.36%~30%[11-12],一年四季均可发病[13],且患病率随年龄增大而升高[14]。目前在世界范围内广泛流行,我国内蒙古、陕西、贵州、新疆、云南和广东等地区均有该病发生的报道[15-16]。但是,临床上内脏型山羊伪结核病临床症状不明显,容易被忽视从而耽误治疗。为了解临床上是否存在Mo和CP混合感染,建立一种同时检测两种病原的PCR方法对上述两种病的监测和防控具有重要意义。【前人研究进展】在Mo和CP两种病原的检测方面,已经建立的方法有PCR、荧光定量PCR、血清学检测方法等。韩瑞鑫等[17]建立了检测Mo的TaqMan实时荧光定量PCR;林裕胜等[18]建立了Mo RPA;王娟等[19]建立了Mo恒温热隔绝式PCR;张双翔等[20]建立了Mo LAMP;赵萍等[21]建立了Mo间接ELISA;马玉馨等[13]建立了CP TaqMan荧光定量PCR;许国洋等[22]建立了CP PMA-PCR;郑敏等[23]、韦志锋等[24]和朱伟英等[25]建立CP PCR;王韡等[26]建立了CP ELISA。【本研究切入点】以上检测方法均为Mo或CP的单一检测方法,相比于同时检测CP和Mo病原的方法效率低。但国内尚未见同时检测CP和Mo 的双重PCR方法相关研究。【拟解决的关键问题】本研究建立Mo和CP双重PCR检测方法可用于临床样品中Mo和CP单一或混合感染的快速检测,为MPGS和羊伪结核病的快速诊断及流行病学调查提供实用方法。
1. 材料与方法
1.1 菌株及临床样品
大肠杆菌(Escherichia coli, Ec)、沙门氏杆菌(Salmonella enteriditis, SE)、金黄色葡萄球菌(Staphylococcus aureus, SA)由福建省农业科学院畜牧兽医研究所禽病研究室和猪病研究室馈赠;山羊支原体山羊亚种(Mycoplasma capricolum subsp. capricolum, Mcc)、山羊支原体山羊肺炎亚种(Mycoplasma capricolum subsp. capripneumoniae, Mccp)由中国农业科学院兰州兽医研究所馈赠;伪结核棒状杆菌(Corynebacterium pseudotuberculosis, CP)[27]、绵羊肺炎支原体(Mycoplasma ovipneumoniae, Mo)[28]、丝状支原体山羊亚种(Mycoplasma mycoides subsp. Capri, Mmc)[29]、山羊莱氏无胆甾原体(Acholeplasma laidlawii, AL)[30]、牛支原体(Mycoplasma bovis, Mb)[31]为本研究室分离、鉴定、保存。70份临床样品于2018年4月至2019年7月采集自福建省三明市(鼻拭子10份、肺组织9份)、宁德市(鼻拭子13份、肺组织7份)和福州市(鼻拭子20份、肺组织11份)的临床上有不同程度流鼻涕、咳嗽、消瘦的病羊。
1.2 主要试剂
LB培养基和DNA提取试剂盒购于生工生物工程(上海)有限公司;改良Frey氏培养基为青岛高科技园海博生物技术有限公司产品;Premix Taq(Ex Taq Version 2.0 plus dye)、DL2000 Marker等购自宝生物工程(大连)有限公司。
1.3 引物设计
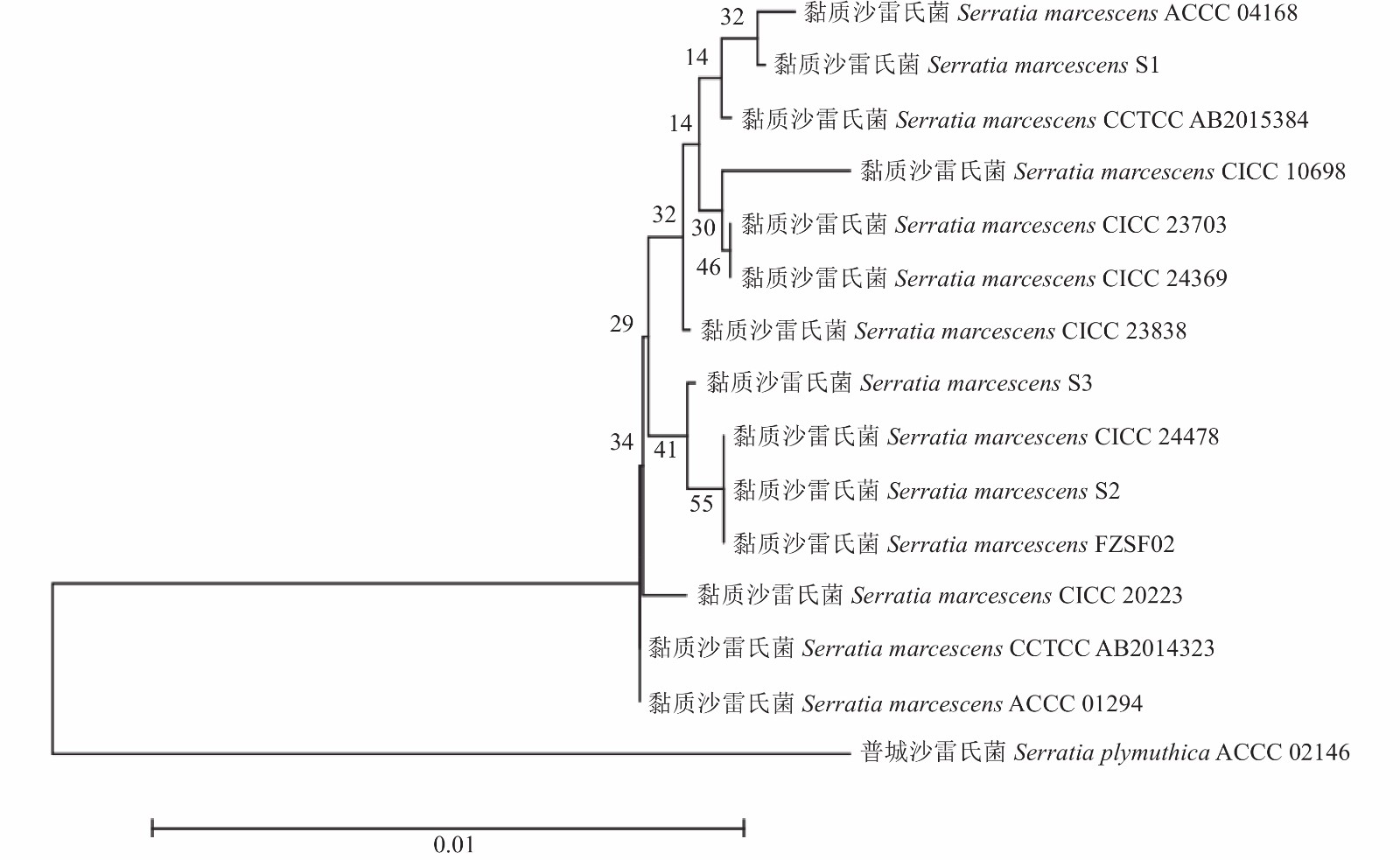
Mo特异性引物F1/R1参照林裕胜等[32]设计;CP特异性引物F2/R2的设计参照许国洋等[22],由铂尚生物技术(上海)有限公司合成,其序列如表1。
表 1 引物序列Table 1. Primer sequence靶基因
Target gene引物名称
Primer name引物序列(5′→ 3′)
Primer sequence(5′→ 3′)扩增片段大小
Amplified fragment size/bpP80基因 F1 GCCTTGGGGTTGGAATTCCTTTGTCTTATTC 705 R1 CATTTGATGCTGAGGTCGGATTTGGACTAAC PLD基因 F2 GTGAGAAGAACCCCGGTATAAG 291 R2 TACCGCACTTATTCTGACACTG 1.4 细菌和支原体核酸的提取
细菌DNA和支原体DNA的提取及浓度测定参考林裕胜等[32]的方法。
1.5 单一PCR反应体系的建立及克隆测序
Mo和CP的单一PCR扩增体系均为20 μL:Premix Taq(Ex Taq Version 2.0 plus dye)10 μL,上、下游引物(10 μmol·L−1)各1 μL,DNA模板2 μL,ddH2O补足至20 μL。PCR反应程序为:94℃预变性5 min;94℃变性45 s,55℃退火45 s,72℃延伸1 min,30个循环;72℃终延伸10 min,4℃保存。经1%琼脂糖凝胶电泳检测PCR产物大小。将Mo和CP的单一PCR扩增产物交铂尚生物技术(上海)有限公司进行克隆测序,将获得的DNA序列与GenBank中的基因序列进行比对。
1.6 双重PCR体系优化
在Mo和CP单一PCR反应的基础上,分别对双重PCR体系中的引物浓度和退火温度进行优化。以Mo和CP混合DNA为模板。优化引物浓度时:两对特异性引物浓度均为10 μmol·L−1,双重 PCR 反应体系20 μL:Premix Taq(Ex Taq Version 2.0 plus dye)10 μL,混合DNA模板2 μL,引物分别为0.7、0.8、0.9、1.0、1.1、1.2 和1.3 μL,ddH2O补足至20 μL;PCR反应程序为:94℃预变性5 min;94℃变性45 s,55℃退火45 s,72℃延伸45 s,30个循环;72℃终延伸10 min,对PCR产物进行1%琼脂糖电泳分析,以确定最佳引物浓度。优化退火浓度时:设置7个温度:51、53、55、57、59、61和63℃,每个温度组加入已优化的引物量,其余条件固定不变进行双重PCR反应,反应结束后经1%琼脂糖凝胶电泳观察结果,以确定最佳退火温度。
1.7 双重PCR特异性试验
分别以Mo+CP、CP、Mo、Mmc、Mcc、Mccp、AL、Mb、SE、Ec、SA的DNA为模板,以ddH2O作为空白对照,按照优化后的双重PCR反应条件进行检测。反应结束后进行1%琼脂糖凝胶电泳观察结果。
1.8 双重PCR灵敏性试验
将已测定浓度的Mo和CP DNA,用ddH2O进行梯度稀释,稀释倍数分别为101、102、103、104、105、106、107、108。分别取2 μL各稀释度的Mo、CP 和Mo+CP的DNA进行单一和双重PCR反应,扩增体系和扩增条件同1.5和1.7,测定该方法的灵敏性。
1.9 双重PCR重复性试验
组内重复试验:取1份Mo+CP、4份Mo、5份CP的DNA样品,以Mccp、Mmc、AL、Ec、SA、SE的DNA样品各1份作阴性对照,以ddH2O作空白对照,每一份样品3个重复,进行双重PCR 扩增试验,扩增体系和扩增条件同1.7;组间重复试验:以上17份样品每间隔3 d分别用双重PCR方法检测1次,共检测3次,PCR扩增体系和扩增条件均同1.7。
1.10 临床样品检测试验
以DNA抽提试剂盒提取70份自福建省不同地区采集的临床样品的DNA,以此DNA为模板进行CP、Mo、Mo+CP检测,重复3次,具体试验方法参考林裕胜等[32]方法。
2. 结果与分析
2.1 Mo和CP单一PCR扩增及扩增产物的鉴定
应用基于Mo的P80基因和CP的PLD基因所设计的2对特异性引物,按照单一PCR反应体系分别进行PCR反应,将扩增得到的目的片段经1%琼脂糖凝胶电泳。电泳结果显示,Mo仅扩增出一条约700 bp左右的目的片段(图略),CP仅扩增出一条约290 bp左右的目的片段(图略),二者均与预期片段大小相符。克隆测序结果显示,所克隆的Mo序列为705 bp,与参考株(GenBank No. KF703747.1)序列同源性为100%;克隆的CP序列为291 bp,与GenBank中收录的山羊(GenBank No. CP039866.1)和绵羊(GenBank No. CP001829.1)伪结核棒状杆菌同源性均为100%。
2.2 双重PCR体系优化结果
经过双重PCR体系优化,确定最佳反应体系为:总体积20 μL,Premix Taq(Ex Taq Version 2.0 plus dye)10 μL,上、下游两对特异性引物(10 μmol·L−1)各1 μL,Mo和CP的DNA混合模板2 μL,ddH2O补足至20 μL;最佳反应程序为:94℃ 5 min;94℃ 45 s,61℃ 45 s,72℃ 45 s,30个循环;72℃ 10 min。以Mo+CP、Mo、CP的 DNA为模板,以Mccp为阴性对照,进行双重PCR扩增。电泳结果显示,Mo+CP混合DNA样品同时扩增出与预期大小相符的约700 bp和290 bp的两个片段,Mo和CP单一DNA分别扩增出约为700 bp和290 bp的单一片段,而阴性对照无条带(图1)。
2.3 双重PCR特异性试验结果
采用优化的双重PCR扩增条件,以Mo+CP、CP、Mo、Mmc、Mcc、Mccp、AL、Mb、SE、Ec、SA的DNA为模板进行PCR扩增,以ddH2O作为空白对照。结果显示,只有Mo+CP、CP、Mo的DNA样品扩增出与预期大小一致的特异性片段,而Mmc、Mcc、Mccp、AL、Mb、SE、Ec、SA、和ddH2O均未扩增出目的片段(图2),表明本研究建立的双重PCR方法特异性好。
2.4 双重PCR灵敏性试验结果
对Mo和CP的DNA进行浓度测定,Mo的DNA质量浓度约为153 ng·μL−1,CP的DNA质量浓度约为35 ng·μL−1。分别以101、102、103、104、105、106、107、108倍稀释,将稀释后的DNA作为模板进行双重PCR扩增。结果显示,当Mo和CP DNA稀释倍数达104和105时,双重PCR仍能扩增出约700 bp和290 bp的两个目的条带(图3),即Mo的检测下限为1 530 pg·μL−1、CP的检测下限为3 500 pg·μL−1,表明该方法的敏感性较高。而单一PCR检测结果显示(图4),Mo的检测灵敏度为15.3 pg·μL−1,CP的检测灵敏度为350 pg·μL−1,分别比双重PCR灵敏低100倍和10倍。
![]() 图 3 双重PCR灵敏性试验注:M: 2 000 DNA Ladder;1: Mo+CP阳性对照;2–9: 分别为101、102、103、104、105、106、107、108倍稀释的含Mo和CP的阳性样本;10: 阴性对照。Figure 3. Sensitivity of multiplex PCRNote:M: 2 000 DNA Ladder; 1: Mo and CP positive control; 2–9: positive specimens containing Mo and CP diluted 101, 102, 103, 104, 105, 106, 107, 108 times, respectively; 10: negative control.
图 3 双重PCR灵敏性试验注:M: 2 000 DNA Ladder;1: Mo+CP阳性对照;2–9: 分别为101、102、103、104、105、106、107、108倍稀释的含Mo和CP的阳性样本;10: 阴性对照。Figure 3. Sensitivity of multiplex PCRNote:M: 2 000 DNA Ladder; 1: Mo and CP positive control; 2–9: positive specimens containing Mo and CP diluted 101, 102, 103, 104, 105, 106, 107, 108 times, respectively; 10: negative control.![]() 图 4 单一PCR灵敏性试验注:A中,M: 2 000 DNA Ladder;1: Mo阳性对照;2–9: 分别为101、102、103、104、105、106、107、108倍稀释的含Mo的阳性样本;10: 阴性对照;B中,M: 2 000 DNA Ladder;1: CP 阳性对照;2–9分别为101、102、103、104、105、106、107、108倍稀释的含CP 的阳性样本;10: 阴性对照。Figure 4. Sensitivity of single PCRNote:A:M: 2 000 DNA Ladder; 1: Mo positive control; 2–9: positive specimens containing Mo diluted 101,102,103,104,105,106,107,108 times, respectively; 10: negative control; B: M: 2 000 DNA Ladder; 1: CP positive control; 2–9: positive specimens containing CP diluted 101,102,103,104,105,106,107,108 times, respectively; 10: negative control.
图 4 单一PCR灵敏性试验注:A中,M: 2 000 DNA Ladder;1: Mo阳性对照;2–9: 分别为101、102、103、104、105、106、107、108倍稀释的含Mo的阳性样本;10: 阴性对照;B中,M: 2 000 DNA Ladder;1: CP 阳性对照;2–9分别为101、102、103、104、105、106、107、108倍稀释的含CP 的阳性样本;10: 阴性对照。Figure 4. Sensitivity of single PCRNote:A:M: 2 000 DNA Ladder; 1: Mo positive control; 2–9: positive specimens containing Mo diluted 101,102,103,104,105,106,107,108 times, respectively; 10: negative control; B: M: 2 000 DNA Ladder; 1: CP positive control; 2–9: positive specimens containing CP diluted 101,102,103,104,105,106,107,108 times, respectively; 10: negative control.2.5 双重PCR重复性试验结果
采用本试验建立的双重PCR方法对Mo + CP、Mo、CP、Mccp、Mmc、AL、Ec、SA、SE的DNA 样品进行3次组内重复试验。结果显示,Mo + CP的DNA样品均扩增出大小约为700 bp和290 bp的2个特异性片段,4份Mo DNA均扩增出大小约为700 bp的特异性片段,5份CP DNA都扩增出大小约为290 bp的特异性片段,其余样品和ddH2O均无扩增片段(图5);组间重复试验结果显示,3次PCR检测结果相同:Mo + CP、Mo、CP的DNA均有目的条带(图略),其余样品均无目的条带,表明该双重PCR重复性好。
2.6 双重PCR临床样品检测结果
应用Mo和CP单一PCR及本研究建立的双重PCR方法分别对来自福州市等不同地区的鼻腔棉拭子样品43份、肺组织27份进行检测。结果显示(表2),Mo和CP单一PCR与双重PCR检测结果完全一致,2种方法的符合率为100%。其中18份为Mo感染,13份为CP感染,5份为Mo和CP的混合感染,且应用Mo和CP单一PCR和双重PCR进行的3次检测结果相同。表明本研究建立的Mo、CP双重 PCR 检测方法可应用于临床样品的快速检测。
表 2 临床样品检测结果Table 2. Detection of pathogens on clinical specimens样品名称
Sample nameMo阳性率
Mo positive rate/%CP阳性率
CP positive rate/%Mo + CP阳性率
Mo + CP positive rate/%鼻拭子
Nasal cotton swab18.60 11.63 4.65 肺组织
Lung tissue37.04 29.63 11.11 所有样品
All samples25.71 18.57 7.14 3. 讨论与结论
随着养羊业的快速发展,羊的疾病日趋复杂化,单一病原引起的感染越来越少,混合感染呈现出上升趋势,对羊病的有效防控难度增加,给养羊业带来了严重的经济损失,已引起广大兽医工作者的关注[33-35]。为了解福建省Mo和CP混合感染情况,有效防治羊病,本研究应用Mo和CP的两对特异性引物,通过优化反应体系和条件以及特异性和灵敏性等试验,建立了Mo和CP的双重PCR检测技术。
双重PCR是指将多对引物同在一个反应体系里,扩增出多个不同大小产物的技术,广泛应用于病原微生物检测与鉴定[36]。双重PCR反应受众多因素的影响,其中影响较大的是退火温度和引物浓度。本试验就引物浓度和退火温度进行优化。优化引物浓度时,设置7个引物梯度(0.7、0.8、0.9、1.0、1.1、1.2 和1.3 μL),其他条件不变,最终确定Mo和CP的最佳引物浓度是上、下游引物(10 μmol·L−1)各1 μL;优化退火温度时,设置7个退火温度(51、53、55、57、59、61和63℃),用最佳引物浓度进行PCR,确定其最佳退火温度为61℃。采用所优化的双重PCR体系仅对Mo和CP有特异性扩增,而对Mmc、Mcc、Mccp、AL、Mb、SE、Ec、SA等病原体的扩增结果均为阴性,与侯宏艳等[37]、张洁[38]、王璇等[39]、李文杨等[9]一般的分离培养鉴定法相比,其特异性更好。该双重PCR方法对Mo和CP的最低检测限分别为1 530 pg·μL−1和3 500 pg·μL−1,表明该方法灵敏度较高;同时具有良好的重复性,可对病原体含量较低的样品进行快速检测。
为了验证该技术的实用效果,采用此方法和单一PCR分别对临床采集的70份样品进行检测。结果检出Mo阳性样品18份(阳性率为25.71%),CP阳性样品13份(阳性率为18.57%),同时感染Mo和CP的阳性样品5份(阳性率为7.14%),Mo、CP单一和双重PCR检测方法的符合率为100%,表明该方法的准确性好,可应用于临床样品的准确快速检测。同时,本次检测结果提示,福建省羊场存在Mo和CP共感染的现象,这与Thomas[40]和许国洋等[41]等的报道相似,应在今后羊病防控工作中引起重视。
-
表 1 供试引物
Table 1 Primes applied
引物 Primes 序列 Sequences(5′-3′) 用途 Purpose 来源 Sources 27F AGAGTTTGATCC TGGCTCAG 16S rDNA 测序 [23] 1492R ACGGTTACCTTGTTACGACTT 16S rDNA 测序 [23] LAD1 ACGATGGACTCCAGAG(G/C/A)N(G/C/A)NNNGGAA 高效热不对称交错PCR [24] LAD3 ACGATGGACTCCAGAG(T/A/C)N(A/G/C)NNNCCAC 高效热不对称交错PCR [24] AC1 ACGATGGACTCCAGAG 高效热不对称交错PCR [24] F389 TCAAGCATTTTATCCGTACTCCTG 高效热不对称交错PCR [24] F536 CGGTTGCATTCGATTCCTGTTTGTA 高效热不对称交错PCR [24] F772 TAGGTTGTATTGATGTTGGACGAG 高效热不对称交错PCR [24] KproF GCAACACTCCGCAATCTATA 菌株ACCC 02146启动子序列 本研究 KproR CTCTATCTCCATGAAAGAGT 菌株ACCC 02146启动子序列 本研究 表 2 菌落形态特征
Table 2 Colony morphology
菌株编号
Strain No.菌落形态特征
Colony morphology characteristicsFZSF02 圆形凸起,湿润黏稠,红色 S1 圆形凸起,湿润黏稠,红色 S2 圆形凸起,湿润黏稠,红色 S3 圆形凸起,湿润黏稠,红色 CICC 23703 圆形凸起,湿润黏稠,深红色 CICC 23838 圆形凸起,湿润黏稠,红色 CICC 24478 圆形凸起,表面干燥粗糙,红色 CICC 10698 圆形凸起,湿润黏稠,橙红色 CICC 20223 圆形凸起,湿润黏稠,橙色 CICC 24369 圆形凸起,湿润黏稠,深红色 CCTCC AB2014323 圆形凸起,湿润黏稠,深红色 CCTCC AB2015384 圆形凸起,湿润黏稠,红色 ACCC 02146 圆形凸起,湿润黏稠,橙红色 ACCC 01294 圆形凸起,湿润黏稠,深红色 ACCC 04168 圆形凸起,湿润黏稠,深红色 表 3 突变体产灵菌红素能力及其菌量
Table 3 Prodigiosin synthesis capabilities and quantity of S.plymuthica mutant
基因登录号
Gene accession number突变体(基因长度/插入位点)
Mutant (gene length/insertion site)/bp功能
FunctionOD600 OD600
改变倍数
OD600 fold changeOD535 OD535改变倍数
OD535 fold
changeOD535改变倍数/
OD600改变倍数
OD535 fold change/
OD600 fold change普城沙雷氏菌 ACCC 02146 2.540 1.000 0.213 1.000 1.000 AEG27540.1 B5-10 (2 673/1 075), B9-2 (2 673/
1 274), B6-4 (2 673/1 075), B6-6
(2 673/792), B5-6 (2 673/792),
B2-11 (2 673/1 266), B5-9 (2 673/153)利用磷酸烯醇式丙酮酸酶(PigC) 2.880 1.134↑ 0.005 0.023↓ 0.020 AEG27539.1 B5-1 (2 601/2 116),B8-1 (2 601/2 096), B7-5 (2 601/1 099), B7-6 (2 601/1 099), B7-11 (2 601/1 338), B9-4 (2 601/2 072), B9-5 (2 601/2 071) 假设蛋白(PigD) 2.644 1.041↑ 0.005 0.023↓ 0.022 AEG27535.1 B7-7 (1 971/1 905), B7-1 (1 971/1 905) 甘氨酸C-乙酰转移酶(PigH) 2.600 1.025↑ 0.015 0.070↓ 0.068 AEG27542.1 B5-12 (1 161/195), B5-13 (1 161/794), B6-8 (1 161/35), B7-8 (1 161/35), B9-1 (1 161/35) 异戊酰基-辅酶A脱氢酶(PigA) 2.731 1.075↑ 0.004 0.019↓ 0.02 AEG27541.1 B5-2 (2 031/1 865), B5-11 (2 031/
1 865), B6-7 (2 031/1 865), B7-12
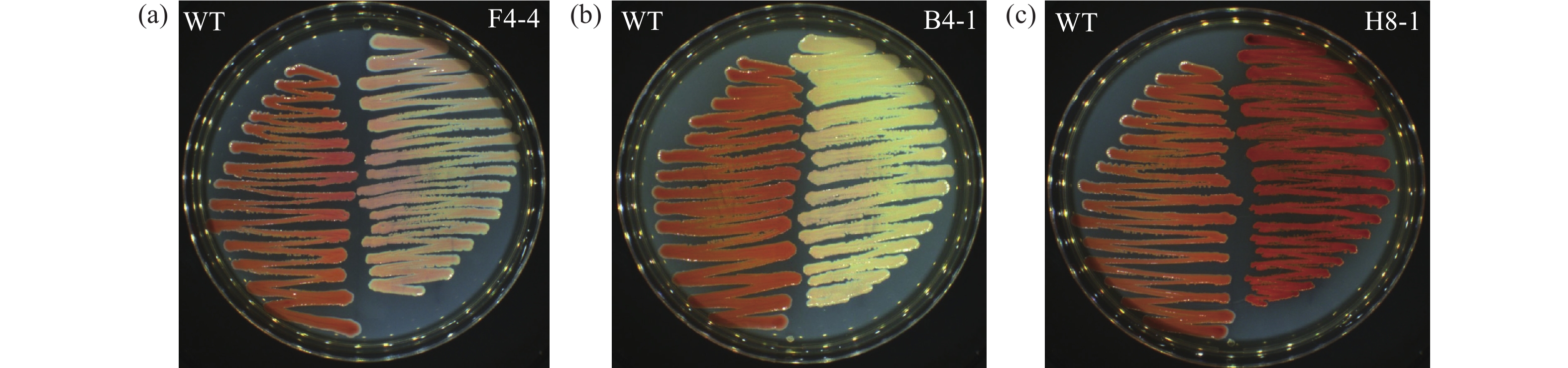
(1 161/35)富马酸还原酶/琥珀酸脱氢酶黄蛋白结构域蛋白(PigB) 2.665 1.049↑ 0.012 0.056↓ 0.053 AEG30298.1 F5-7 (558/202), F5-4 (558/524), F5-10 (558/273), F5-5 (558/518), F5-6 (558/495), F5-9 (558/189) 麦芽糖O-乙酰转移酶 2.618 1.031↑ 0.037 0.174↓ 0.169 AEG29267.1 H8-8 (978/910), H8-9 (978/262), H9-8 (978/217) H-醌氧化还原酶亚基1 2.355 0.927↓ 0.245 1.150↑ 1.241 AEG27501.1 H7-1 (1 011/842), H7-4 (1 011/425), H9-3 (1 011/378), H9-6 (1 011/234) 二氢乳清酸脱氢酶 1.967 0.774↓ 0.589 2.765↑ 3.572 AEG29268.1 H9-8 (2 748/217), H7-5 (2 748/2 682), H7-9 (2 748/2 041) NADH-醌氧化还原酶G链 2.216 0.872↓ 0.243 1.140↑ 1.307 AEG26904.1 B9-3 (495/290), B7-2 (495/290), B7-3 (495/290) 肽基脯氨酰异构酶B 3.827 1.507↑ 0.008 0.038↓ 0.025 AEG26553.1 H8-12 (1 800/1 017), H7-3 (1 800/1 236) 亚硫酸盐还原酶(NADPH)黄蛋白α组分 2.646 1.042↑ 0.269 1.263↑ 1.212 AEG29408.1 H9-5 (1 728/1 484), H7-11 (1 728/1 484) 磷酸烯醇丙酮酸蛋白磷酸转移酶Ptsl 2.660 1.047↑ 0.574 2.695↑ 2.574 AEG29263.1 H8-4 (1 848/189), H9-1 (1 848/853) NADH-醌氧化还原酶亚基L 2.473 0.974↓ 0.231 1.085↑ 1.114 AEG27032.1 H6-5 (390/192) 琥珀酸脱氢酶,细胞色素b556亚基 1.758 0.692↓ 0.230 1.080↑ 1.561 AEG29294.1 H9-10 (1 518/1 085) 氨基磷酸核糖转移酶 2.317 0.912↓ 0.273 1.282↑ 1.406 AEG27629.1 H9-9 (1 047/533) 二氢乳清酸酶 2.164 0.852↓ 0.231 1.085↑ 1.273 AEG27038.1 H8-3 (1 167/336) 琥珀酰辅酶a合成酶(ADP形成)β亚基 2.230 0.878↓ 0.282 1.324↑ 1.508 AEG27031.1 H8-5 (1 293/402), H8-2 (1 293/0) 柠檬酸合成酶I 2.052 0.808↓ 0.333 1.563↑ 1.934 AEG29271.1 H8-7 (1 797/618) NADH-醌氧化还原酶亚基C/D 2.542 1.001↑ 0.293 1.376↑ 1.375 AEG26076.1 H7-7 (1 284/606) 磷酸核糖胺-甘氨酸连接酶 2.579 1.015↑ 0.239 1.122↑ 1.105 AEG30581.1 F4-1 (1 785/73) ATP结合蛋白 2.640 1.040↑ 0.052 0.244↓ 0.235 AEG26238.1 B5-3 (630/624) 假设蛋白ALQ63_03 824 1.416 0.557↓ 0.01 0.047↓ 0.084 AEG29272.1 H7-6 (675/317) H-醌氧化还原酶亚基K 2.342 0.922↓ 0.285 1.338↑ 1.451 AEG27034.1 H7-8 (1 710/1 665), H9-7 (1 710 /1 352) 琥珀酸脱氢酶或富马酸还原酶黄蛋白亚基 1.603 0.631↓ 0.055 0.258↓ 0.409 AEG27939.1 B4-1 (435/109), B6-3 (435/109), B6-5 (435/108) MarR家族转录调控因子SlyA 2.712 1.068↑ 0.006 0.028↓ 0.026 AEG28365.1 F4-4 (849/38), F4-2 (849/36), F4-3 (849/38) winged helix家族双组分转录调控因子RstA 2.71 1.067↑ 0.039 0.183↓ 0.172 AEG30425.1 H8-1 (633/262) cAMP激活的全局转录调控因子CRP,转录调控因子Crp/Fnr家族 2.243 0.883↓ 0.860 4.038↑ 4.573 AEF52 157.1 H7-14 (267/3) FAD装配因子SdhE 2.737 1.078↑ 0.259 1.216↑ 1.128 AEG27490.1 H7-10 (1 110/1 089) 孔蛋白OmpC 1.460 0.575↓ 0.585 2.746↑ 4.776 AEG26866.1 F5-12 (1 188/864) 多药外排RND转运蛋白质周适配器亚单位SdeX 2.910 1.146↑ 0.022 0.103↓ 0.090 AEG29409.1 H3-1 (510/364) 葡萄糖转运蛋白亚基IIA_ 2.234 0.880↓ 0.051 0.239↓ 0.272 下划线标注的为代表性突变体;“↑”代表升高;“↓”代表降低。
Underline indicates mutant; "↑" indicates elevation; "↓" indicates decline. -
[1] PANDEY R, CHANDER R, SAINIS K B. A novel prodigiosin-like immunosuppressant from an alkalophilic Micrococcus sp. [J]. International Immunopharmacology, 2003, 3(2): 159−167. DOI: 10.1016/S1567-5769(02)00114-5
[2] 朱雄伟, 徐智鹏, 张楠, 等. 粘质沙雷氏菌代谢产物灵菌红素的鉴定 [J]. 化学与生物工程, 2012, 29(11):80−82. ZHU X W, XU Z P, ZHANG N, et al. Identification of metabolite prodigiosin of Serratia marcescens [J]. Chemistry & Bioengineering, 2012, 29(11): 80−82.(in Chinese)
[3] 袁保红, 杜青平, 蔡创华, 等. 海洋细菌Pseudomonas sp. 色素的提取及稳定性的研究 [J]. 海洋通报, 2005, 24(6):92−96. YUAN B H, DU Q P, CAI C H, et al. Study on the extraction and stability of pigments from a marine bacterium Pseudomonas sp [J]. Marine Science Bulletin, 2005, 24(6): 92−96.(in Chinese)
[4] 傅奇. 灵菌红素产生菌的筛选鉴定及其发酵条件优化[D]. 南昌: 南昌大学, 2011. FU Q. Screening and identification of prodigiosin-producing bacteria and optimization of fermentation conditions[D]. Nanchang: Nanchang University, 2011. (in Chinese)
[5] JÉRSIA ARAÚJO A, MARINHO FILHO J D B, SOUSA T S, et al. Evidences for the involvement of HER on prodigiosin anticancer effects [J]. Planta Medica, 2012, 78(11): 78−92.
[6] ZHAO C, QIU S Z, HE J, et al. Prodigiosin impairs autophagosome-lysosome fusion that sensitizes colorectal cancer cells to 5-fluorouracil-induced cell death [J]. Cancer Letters, 2020, 481: 15−23. DOI: 10.1016/j.canlet.2020.03.010
[7] D'ALESSIO R, BARGIOTTI A, CARLINI O, et al. Synthesis and immunosuppressive activity of novel prodigiosin derivatives [J]. Journal of Medicinal Chemistry, 2000, 43(13): 2557−2565. DOI: 10.1021/jm001003p
[8] 王玉洁, 孙诗清, 朱长俊, 等. 天然红色素灵菌红素的抗菌性能及应用 [J]. 天然产物研究与开发, 2012, 24(11):1626−1629,1654. DOI: 10.3969/j.issn.1001-6880.2012.11.027 WANG Y J, SUN S Q, ZHU C J, et al. Antibacterial property and application of natural red pigment prodigiosin [J]. Natural Product Research and Development, 2012, 24(11): 1626−1629,1654.(in Chinese) DOI: 10.3969/j.issn.1001-6880.2012.11.027
[9] NAKASHIMA T, YAMAGUCHI K, ODA T, et al. Evaluation of the anti-Trichophyton activity of a prodigiosin analogue produced by γ-proteobacterium, using stratum corneum epidermis of the Yucatan micropig [J]. Journal of Infection and Chemotherapy, 2005, 11(3): 123−128. DOI: 10.1007/s10156-005-0376-0
[10] KANCHARLA P, LI Y X, YELUGURI M, et al. Total synthesis and antimalarial activity of 2-(p-hydroxybenzyl)-prodigiosins, isoheptylprodigiosin, and geometric isomers of tambjamine MYP1 isolated from marine bacteria [J]. Journal of Medicinal Chemistry, 2021, 64(12): 8739−8754. DOI: 10.1021/acs.jmedchem.1c00748
[11] GENES C, BAQUERO E, ECHEVERRI F, et al. Mitochondrial dysfunction in Trypanosoma cruzi: The role of Serratia marcescens prodigiosin in the alternative treatment of Chagas disease [J]. Parasites & Vectors, 2011, 4(1): 66.
[12] KRAMAR A, ILIC-TOMIC T, PETKOVIC M, et al. Crude bacterial extracts of two new Streptomyces sp. isolates as bio-colorants for textile dyeing [J]. World Journal of Microbiology and Biotechnology, 2014, 30(8): 2231−2240. DOI: 10.1007/s11274-014-1644-x
[13] JEONG H, YIM J H, LEE C, et al. Genomic blueprint of Hahella chejuensis, a marine microbe producing an algicidal agent [J]. Nucleic Acids Research, 2005, 33(22): 7066−7073. DOI: 10.1093/nar/gki1016
[14] ZHANG H J, WANG H, ZHENG W, et al. Toxic effects of prodigiosin secreted by Hahella sp. KA22 on harmful Alga Phaeocystis globosa [J]. Frontiers in Microbiology, 2017, 8: 999. DOI: 10.3389/fmicb.2017.00999
[15] WILLIAMS R P, GOLDSCHMIDT M E, GOTT C L. Inhibition by temperature of the terminal step in biosynthesis of prodigiosin [J]. Biochemical and Biophysical Research Communications, 1965, 19(2): 177−181. DOI: 10.1016/0006-291X(65)90500-0
[16] ZHANG F, WEI Q E, TONG H, et al. Crystal structure of MBP-PigG fusion protein and the essential function of PigG in the prodigiosin biosynthetic pathway in Serratia marcescens FS14 [J]. International Journal of Biological Macromolecules, 2017, 99: 394−400. DOI: 10.1016/j.ijbiomac.2017.02.088
[17] PAN X W, SUN C H, TANG M, et al. LysR-type transcriptional regulator MetR controls prodigiosin production, methionine biosynthesis, cell motility, H2O2 tolerance, heat tolerance, and exopolysaccharide synthesis in Serratia marcescens [J]. Applied and Environmental Microbiology, 2020, 86(4): e02241−e02219.
[18] WEI Y H, CHEN W C. Enhanced production of prodigiosin-like pigment from Serratia marcescens SMdeltaR by medium improvement and oil-supplementation strategies [J]. Journal of Bioscience and Bioengineering, 2005, 99(6): 616−622. DOI: 10.1263/jbb.99.616
[19] KHAYYAT AHDAB N, ABBAS HISHAM A, KHAYAT MAAN T, et al. Secnidazole is a promising imidazole mitigator of Serratia marcescens virulence [J]. Microorganisms, 2021, 9(11): 2333. DOI: 10.3390/microorganisms9112333
[20] SHANKS R M Q, STELLA N A, LAHR R M, et al. Suppressor analysis of eepR mutant defects reveals coordinate regulation of secondary metabolites and serralysin biosynthesis by EepR and HexS [J]. Microbiology (Reading, England), 2017, 163(2): 280−288. DOI: 10.1099/mic.0.000422
[21] LEE C M, MONSON R E, ADAMS R M, et al. The LacI-family transcription factor, RbsR, is a pleiotropic regulator of motility, virulence, siderophore and antibiotic production, gas vesicle morphogenesis and flotation in Serratia [J]. Frontiers in Microbiology, 2017, 8: 1678. DOI: 10.3389/fmicb.2017.01678
[22] GRISTWOOD T, MCNEIL M B, CLULOW J S, et al. PigS and PigP regulate prodigiosin biosynthesis in Serratia via differential control of divergent operons, which include predicted transporters of sulfur-containing molecules [J]. Journal of Bacteriology, 2011, 193(5): 1076−1085. DOI: 10.1128/JB.00352-10
[23] 刘径, 张珂恒, 曾永三. 昆虫病原线虫Oscheius myriophila共生细菌菌株B1的分离与鉴定 [J]. 广东农业科学, 2016, 43(3):111−115. LIU J, ZHANG K H, ZENG Y S. Isolation and identification of a symboiotic bacterial strain (B1) from an entomopathogenic nematode, Oscheius myriophila [J]. Guangdong Agricultural Sciences, 2016, 43(3): 111−115.(in Chinese)
[24] JIA X B, LIN X J, CHEN J C. Linear and exponential TAIL-PCR: A method for efficient and quick amplification of flanking sequences adjacent to Tn5 transposon insertion sites [J]. AMB Express, 2017, 7(1): 195. DOI: 10.1186/s13568-017-0495-x
[25] LIU Y G, CHEN Y L. High-efficiency thermal asymmetric interlaced PCR for amplification of unknown flanking sequences[J]. BioTechniques, 2007, 43(5): 649−656.
[26] JIA X B, LIU F C, ZHAO K, et al. Identification of essential genes associated with prodigiosin production in Serratia marcescens FZSF02 [J]. Frontiers in Microbiology, 2021, 12: 705853. DOI: 10.3389/fmicb.2021.705853
[27] 刘方晨, 贾宪波, 吴良泉, 等. 黏质沙雷氏菌灵菌红素合成基因簇异源表达及其潜在的温度调控机制 [J]. 福建农业学报, 2021, 36(3):337−344. LIU F C, JIA X B, WU L Q, et al. Heterologous expression and temperature regulation of prodigiosin-synthesis gene cluster in Serratia marcecens [J]. Fujian Journal of Agricultural Sciences, 2021, 36(3): 337−344.(in Chinese)
[28] WEATHERSPOON-GRIFFIN N, YANG D Z, KONG W, et al. The CpxR/CpxA two-component regulatory system up-regulates the multidrug resistance cascade to facilitate Escherichia coli resistance to a model antimicrobial peptide [J]. The Journal of Biological Chemistry, 2014, 289(47): 32571−32582. DOI: 10.1074/jbc.M114.565762
[29] GRISTWOOD T, FINERAN P C, EVERSON L, et al. The PhoBR two-component system regulates antibiotic biosynthesis in Serratia in response to phosphate [J]. BMC Microbiology, 2009, 9: 112. DOI: 10.1186/1471-2180-9-112
[30] STELLA N A, LAHR R M, BROTHERS K M, et al. Serratia marcescens cyclic AMP receptor protein controls transcription of EepR, a novel regulator of antimicrobial secondary metabolites [J]. Journal of Bacteriology, 2015, 197(15): 2468−2478. DOI: 10.1128/JB.00136-15
[31] HORNG Y T, CHANG K C, LIU Y N, et al. The RssB/RssA two-component system regulates biosynthesis of the tripyrrole antibiotic, prodigiosin, in Serratia marcescens [J]. International Journal of Medical Microbiology, 2010, 300(5): 304−312. DOI: 10.1016/j.ijmm.2010.01.003
[32] FINERAN P C, SLATER H, EVERSON L, et al. Biosynthesis of tripyrrole and beta-lactam secondary metabolites in Serratia: Integration of quorum sensing with multiple new regulatory components in the control of prodigiosin and carbapenem antibiotic production [J]. Molecular Microbiology, 2005, 56(6): 1495−1517. DOI: 10.1111/j.1365-2958.2005.04660.x
[33] QIU S S, JIA S S, ZHANG F, et al. Two component system CpxR/a regulates the prodigiosin biosynthesis by negative control in Serratia marcescens FS14 [J]. Biochemical and Biophysical Research Communications, 2021, 579: 136−140. DOI: 10.1016/j.bbrc.2021.09.050
[34] LI Y Q, CEN P L, CHEN S F, et al. A pair of two-component regulatory genes ecrA1/A2 in S. coelicolor [J]. Journal of Zhejiang University-SCIENCE A, 2004, 5(2): 173−179. DOI: 10.1631/jzus.2004.0173
[35] WILLIAMSON N R, FINERAN P C, OGAWA W, et al. Integrated regulation involving quorum sensing, a two-component system, a GGDEF/EAL domain protein and a post-transcriptional regulator controls swarming and RhlA-dependent surfactant biosynthesis in Serratia [J]. Environmental Microbiology, 2008, 10(5): 1202−1217. DOI: 10.1111/j.1462-2920.2007.01536.x
[36] 张亚. 粘质沙雷氏菌BaeS胞外感受器结构域晶体结构及双组份系统BaeS/R功能的研究[D]. 南京: 南京农业大学, 2016. ZHANG Y. Study on crystal structure of BaeS extracellular receptor domain of Serratia marcescens and BaeS/R function of two-component system[D]. Nanjing: Nanjing Agricultural University, 2016. (in Chinese)
[37] 贾宪波, 刘方晨, 赵恪, 等. 粘质沙雷氏菌FZSF02中转录调控因子OmpR的生物学功能 [J]. 福建农业学报, 2021, 36(12):1491−1498. JIA X B, LIU F C, ZHAO K, et al. Biological functions of transcription factor OmpR in Serratia marcescens FZSF02 [J]. Fujian Journal of Agricultural Sciences, 2021, 36(12): 1491−1498.(in Chinese)
[38] LERY L M S, GOULART C L, FIGUEIREDO F R, et al. A comparative proteomic analysis of Vibrio cholerae O1 wild-type cells versus a phoB mutant showed that the PhoB/PhoR system is required for full growth and rpoS expression under inorganic phosphate abundance [J]. Journal of Proteomics, 2013, 86: 1−15. DOI: 10.1016/j.jprot.2013.04.038
[39] HARRIS A K P, WILLIAMSON N R, SLATER H, et al. The Serratia gene cluster encoding biosynthesis of the red antibiotic, prodigiosin, shows species- and strain-dependent genome context variation[J]. Microbiology (Reading, England), 2004, 150(Pt 11): 3547-3560.
[40] THOMSON N R, COX A, BYCROFT B W, et al. The Rap and Hor proteins of Erwinia, Serratia and Yersinia: A novel subgroup in a growing superfamily of proteins regulating diverse physiological processes in bacterial pathogens [J]. Molecular Microbiology, 1997, 26(3): 531−544. DOI: 10.1046/j.1365-2958.1997.5981976.x
[41] XIANG T T, ZHOU W, XU C L, et al. Transcriptomic analysis reveals competitive growth advantage of non-pigmented Serratia marcescens mutants [J]. Frontiers in Microbiology, 2022, 12: 793202. DOI: 10.3389/fmicb.2021.793202
[42] GREEN J, SCOTT C, GUEST J R. Functional versatility in the CRP-FNR superfamily of transcription factors: FNR and FLP[M]//Advances in Microbial Physiology. Amsterdam: Elsevier, 2001: 1-34.
[43] SUN D, ZHOU X G, LIU C, et al. Fnr negatively regulates prodigiosin synthesis in Serratia sp. ATCC 39006 during aerobic fermentation [J]. Frontiers in Microbiology, 2021, 12: 734854. DOI: 10.3389/fmicb.2021.734854
[44] 刘星, 王希东, 刘君. 西瓜食酸菌RND蛋白家族外排转运体cusB基因抗铜功能研究 [J]. 微生物学通报, 2016, 43(1):97−106. LIU X, WANG X D, LIU J. Functional analysis of a RND family effiux transporter component-cusB gene associated with copper resistance in Acidovorax citrulli [J]. Microbiology China, 2016, 43(1): 97−106.(in Chinese)
[45] GRISTWOOD T, FINERAN P C, EVERSON L, et al. PigZ, a TetR/AcrR family repressor, modulates secondary metabolism via the expression of a putative four-component resistance-nodulation-cell-division efflux pump, ZrpADBC, in Serratia sp. ATCC 39006 [J]. Molecular Microbiology, 2008, 69(2): 418−435. DOI: 10.1111/j.1365-2958.2008.06291.x
[46] MCNEIL M B, CLULOW J S, WILF N M, et al. SdhE is a conserved protein required for flavinylation of succinate dehydrogenase in bacteria [J]. Journal of Biological Chemistry, 2012, 287(22): 18418−18428. DOI: 10.1074/jbc.M111.293803
[47] ESCHENBRENNER M, COVÈS J, FONTECAVE M. The flavin reductase activity of the flavoprotein component of sulfite reductase from Escherichia coli [J]. Journal of Biological Chemistry, 1995, 270(35): 20550−20555. DOI: 10.1074/jbc.270.35.20550
[48] RADZIG M A, KOKSHAROVA O A, KHMEL’ I A. Antibacterial effects of silver ions on growth of gram-negative bacteria and biofilm formation [J]. Molecular Genetics, Microbiology and Virology, 2009, 24(4): 194−199. DOI: 10.3103/S0891416809040065
[49] BEGIC S, WOROBEC E A. Site-directed mutagenesis studies to probe the role of specific residues in the external loop (L3) of OmpF and OmpC porins in susceptibility of Serratia marcescens to antibiotics [J]. Canadian Journal of Microbiology, 2007, 53(6): 710−719. DOI: 10.1139/W07-018
[50] BRANDT U. Energy converting NADH: Quinone oxidoreductase (complex I) [J]. Annual Review of Biochemistry, 2006, 75: 69−92. DOI: 10.1146/annurev.biochem.75.103004.142539
[51] SCHULER F, YANO T, DI BERNARDO S, et al. NADH-quinone oxidoreductase: PSST subunit couples electron transfer from iron–sulfur cluster N2 to quinone [J]. Proceedings of the National Academy of Sciences of the United States of America, 1999, 96(7): 4149−4153. DOI: 10.1073/pnas.96.7.4149
-
期刊类型引用(4)
1. 杨帅,段宏伟,吕建树,曾建林,闫振兴,胡俊杰,张勇,赵兴绪. 甘肃省庆阳地区湖羊皮下脓肿病的流行病学调查及病原体分析. 核农学报. 2023(12): 2510-2517 .  百度学术
百度学术
2. 杨鹏,吴燕,岳筠,陈静,李梅,王慧,张双翔,文明,程振涛. 绵羊肺炎支原体P113蛋白C末端基因真核表达载体的构建及其小鼠免疫应答. 中国兽医学报. 2022(03): 496-501+521 .  百度学术
百度学术
3. 杨鹏,杨源,岳筠,陈静,王慧,朱二鹏,张双翔,文明,程振涛. 绵羊肺炎支原体感染对贵州不同品种山羊肺脏和血液Toll样受体基因转录水平的影响. 动物医学进展. 2022(12): 1-9 .  百度学术
百度学术
4. 尹峥,刘刚,王晶晶,李晨露,徐海玲,张琪,许信刚. 基于PLD蛋白的伪结核棒状杆菌血清抗体间接ELISA检测方法的建立与应用. 中国兽医科学. 2021(01): 9-16 .  百度学术
百度学术
其他类型引用(4)




 下载:
下载: