Formation of Proteus mirabilis Persister in Chicken under Gentamicin Sulphate Treatment
-
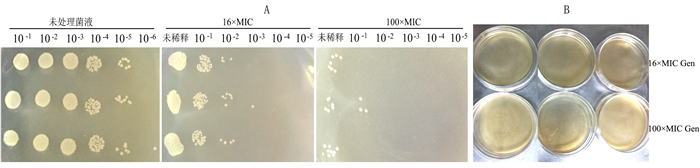
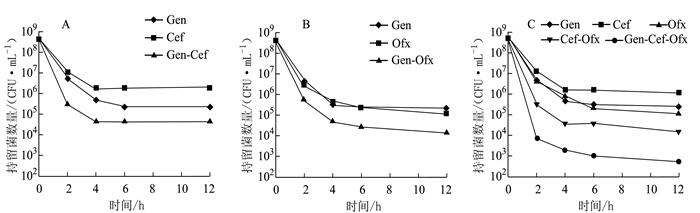
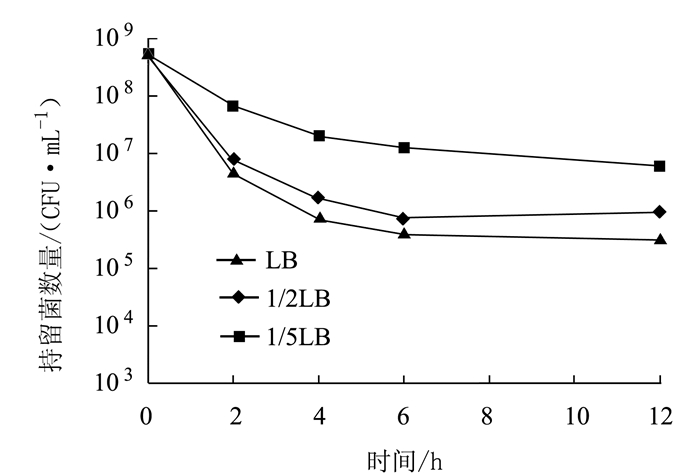
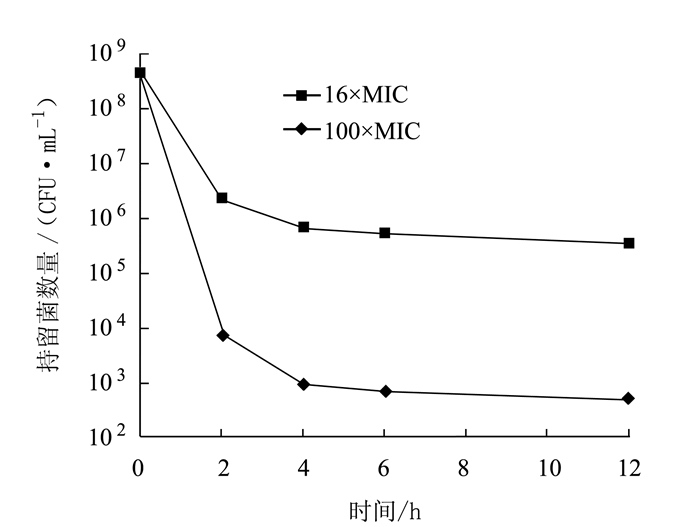
摘要: 为了解奇异变形杆菌持留菌形成特征和防控家禽养殖中细菌性疾病,以鸡源致病菌奇异变形杆菌PM2658为研究对象,通过二倍稀释法测得抗生素对鸡源奇异变形杆菌PM2658的最小抑菌浓度(Minimum inhibitory of concentration,MIC),用斑点培养(spot-plate)法分析PM2658在不同抗生素和营养条件下持留菌形成特征。结果显示,硫酸庆大霉素、头孢噻肟钠、氧氟沙星对PM2658的MIC值分别为1.56、0.78和3.13 μg·mL-1。在16×MIC和100×MIC硫酸庆大霉素处理下,鸡源奇异变形杆菌PM2658都可以形成持留菌,形成率分别为8.04×10-4和1.03×10-6。16×MIC硫酸庆大霉素-4×MIC头孢噻肟钠、16×MIC硫酸庆大霉素-4×MIC氧氟沙星、4×MIC头孢噻肟钠-4×MIC氧氟沙星、16×MIC硫酸庆大霉素-4×MIC头孢噻肟钠-4×MIC氧氟沙星不同抗生素组合处理下,鸡源奇异变形杆菌PM2658的持留菌形成规律与单独硫酸庆大霉素处理的持留菌形成规律一致,也表明鸡源奇异变形杆菌PM2658持留菌具有多重抗生素耐受性。在16×MIC硫酸庆大霉素处理下,与LB和1/2 LB培养条件相比,1/5 LB培养条件下鸡源奇异变形杆菌持留菌形成率最高,比前两者分别提高了17.67倍和5.05倍,说明鸡源奇异变形杆菌持留菌的形成还与营养环境密切相关。持留菌遗传学特性检测结果显示,鸡源奇异变形杆菌PM2658持留菌的遗传学特性并没有发生改变。鸡源奇异变形杆菌确实可以形成持留菌,其形成特征取决于特定的抗生素条件。Abstract: For understanding the biological characteristics of persister formation of Proteus mirabilis, and control and prevention of bacterial diseases in poultry, Proteus mirabilis PM2658 from chicken was used as materials. Minimum inhibitory of concentrations (MIC) of antibiotics to Proteus mirabilis PM2658 were determined by two-fold dilution method. Under different antibiotic treatments and nutritional conditions, the characteristics of persister formation of Proteus mirabilis were determined by spot-plate protocol. Results showed that MIC of gentamicin sulphate (Gen), cefotaxime sodium (Cef) and ofloxacin (Ofx) to Proteus mirabilis PM2658 were 1.56, 0.78 and 3.13 μg·mL-1, respectively. Treated by 16×MIC and 100×MIC of Gen, stationary phase of Proteus mirabilis PM2658 could form persister, and the percentages of persister formation were 8.04×10-4 and 1.03×10-6, respectively. Moreover, the regularity of peresister formation under antibiotic combinations of 16×MIC Gen-4×MIC Cef, 16×MIC Gen-4×MIC Ofx, 4×MIC Cef-4×MIC Ofx, 16×MIC Gen-4×MIC Cef-4×MIC Ofx was the same as that of single Gen treatment, indicating that persister of Proteus mirabilis PM2658 had multiple drug tolerance. The rate of formation of Proteus mirabilis PM2658 persister in 1/5 LB was higher than those in LB and 1/2 LB under 16×MIC Gen treatment, which separately increased by 17.67 times and 5.05 times, which indicated that the formation of Proteus mirabils PM2658 persister was related to nutrition of environment. The heritage of Proteus mirabilis PM2658 persister did not change, which was further determined after every different treatments mentioned above. Taken together, Proteus mirabilis from chicken do form persister, and characteristics of persister formation depends on specific antibiotic.
-
Keywords:
- Proteus mirabilis /
- drug tolerance /
- persister /
- gentamicin sulphate
-
-
-
[1] BIGGER J W. Treatment of staphylococcal infections with penicillin by intermittent sterilization[J]. Lancet, 1944, 244(6320):497-500. DOI: 10.1016/S0140-6736(00)74210-3
[2] COHEN N R, LOBRITZ M A, COLLINS J J. Microbial persistence and the road to drug resistance[J]. Cell Host Microbe, 2013, 13(6):632-642. DOI: 10.1016/j.chom.2013.05.009
[3] ADAMS K N, TAKAKI K, CONNOLLY L E, et al. Drug tolerance in replicating mycobacteria mediated by a macrophage-induced efflux mechanism[J]. Cell, 2011, 145(1):39-53. DOI: 10.1016/j.cell.2011.02.022
[4] CHONG Y P, PARK S J, KIM H S, et al. Persistent Staphylococcus aureus bacteria:a prospective analysis of risk factors, outcomes, and microbiologic and genotypic characteristics of isolates[J]. Medicine (Baltimore), 2013, 92(2):98-108. DOI: 10.1097/MD.0b013e318289ff1e
[5] HOFSTEENGE N, NIMWEGEN E V, SILANDER O K. Quantitative analysis of persister fractions suggests different mechanisms of formation among environmental isolates of E.coli[J]. BMC Microbiology, 2013, 13:25. DOI: 10.1186/1471-2180-13-25
[6] HOLLING N, DEDI C, JONES C E, et al. Evaluation of environmental scanning electron microscopy for analysis of Proteus mirabilis crystalline biofilms in situ on urinary catheters[J]. FEMS Microbiology Letters, 2014, 355(4):20-27. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=JJ0233660810
[7] BORGMAN C J. Proteus mirabilis and its role in dacryocystitis[J]. Optometry and Vision Science, 2014, 91(9):230-235. DOI: 10.1097/OPX.0000000000000347
[8] 周芳, 冉丹丹, 刘飞, 等.鸡源奇异变形杆菌的分离鉴定及系统进化分析[J].动物医学进展, 2015, 36(7):29-32. DOI: 10.3969/j.issn.1007-5038.2015.07.006 [9] 李欣南, 韩镌竹, 宁宜宝.鸡源奇异变形杆菌的分离鉴定及耐药性分析[J].黑龙江畜牧兽医, 2015, (6):165-167, 289. http://www.cnki.com.cn/Article/CJFDTOTAL-HLJX201511055.htm [10] CLINICAL AND LABORATORY STANDARDS INSTITUTE. Reference method for broth dilution antifungal susceptibility testing of yeasts-second edition:approved standard M27-A[M]. Wayne, PA:CLSI, 2002.
[11] BUTT A, HIGMAN V A, WILLIAMS C, et al. The HicA toxin from Burkholderia pseudomallei has a role in persister cell formation[J]. The Biochemical Journal, 2014, 459(2):333-344. DOI: 10.1042/BJ20140073
[12] 胡丽庆, 史煜波, 孙定河, 等.奇异变形杆菌耐药性的4年监测及碳青霉烯类耐药株的耐药机制研究[J].中国微生态学杂志, 2012, 24(7):611-614. http://d.old.wanfangdata.com.cn/Periodical/zgwstxzz201207010 [13] PEARSON M M, SEBAIHIA M, CHURCHER C, et al. Complete genome sequence of uropathogenic Proteus mirabilis, a master of both adherence and motility[J]. Journal of Bacteriology, 2008, 190(11):4027-4037. DOI: 10.1128/JB.01981-07
[14] GRANT S S, HUNG D T. Persistence bacterial infections, antibiotic tolerance, and the oxidative stress response[J]. Virulence, 2013, 4(4):273-283. DOI: 10.4161/viru.23987
[15] GUSAROV I, SHATALIN K, STARODUBTSEVA M, et al. Endogenous nitric oxide protects bacteria against a wide spectrum of antibiotics[J]. Science, 2009, 325(5964):1380-1384. DOI: 10.1126-science.1175439/
[16] LIU Y, IMLAY J A. Cell death from antibiotics without the involvement of reactive oxygen species[J]. Science, 2013, 339(6124):1210-1213. DOI: 10.1126/science.1232751
[17] FENG J, WEITNER M, SHI WL, et al. Eradication of biofilm-like microcolony structures of Borrelia burgdorferi by daunomycin and daptomycin but not mitomycin C in combination with doxycycline and cefuroxime[J]. Front Microbiol, 2016, 7:62. http://pubmedcentralcanada.ca/pmcc/articles/PMC4748043/
[18] PU Y Y, ZHAO Z L, LI Y X, et al. Enhanced efflux activity facilitates drug tolerance in dormant bacterial cells[J]. Mol Cell, 2016, 62(2):284-294. DOI: 10.1016/j.molcel.2016.03.035
[19] BALABAN, N Q, MERRIN J, CHAIT R, et al. Bacterial persistence as a phenotypic switch[J]. Science, 2004, 305(5690):1622-1625. DOI: 10.1126/science.1099390
[20] KORCH S B, HILL T M. Ectopic overexpression of wild-type and mutant hipA genes in Escherichia coli:effects on macromolecular synthesis and persister formation[J]. Journal of Bacteriology, 2006, 188(11):3826-3836. DOI: 10.1128/JB.01740-05
[21] FUNG D K, CHAN E W, CHIN M L. Delineation of a bacterial starvation stress response network which can mediate antibiotic tolerance development[J]. Antimicrobial Agents and Chemotherapy, 2010, 54(3):1082-1093. DOI: 10.1128/AAC.01218-09
[22] NGUYEN D, JOSHI-DATAR A, LEPINE F, et al. Active starvation response mediate antibiotic tolerance in biofilms and nutrient-limited bacteria[J]. Science, 2011, 334(6058):982-986. DOI: 10.1126/science.1211037




 下载:
下载: