Response Surface Optimization of ISSR-PCR Reaction for Genetic Study on Phyllanthus Emblica
-
摘要:目的 优化余甘子种质资源ISSR-PCR反应体系,为余甘子种质资源遗传多样性及亲缘关系研究提供基础。方法 以缅甸、印度、广东、云南、福建等5份来源不同的余甘子种质资源的基因组DNA组成混合的DNA模板,综合单因素试验和响应面分析法,分析引物浓度、2×Taq Master Mix添加量、DNA模板量、退火温度等反应条件对ISSR-PCR反应体系的影响,优化建立余甘子ISSR-PCR反应体系。结果 引物浓度和2×Taq Master Mix添加量对扩增效果有较大影响,DNA模板量影响较小;引物浓度和DNA模板量交互作用明显;余甘子ISSR反应体系为引物浓度0.4 μmol·L−1,2×Taq Master Mix添加量13 μL,DNA模板量30 ng,扩增结果与响应面分析模型理论值相对误差仅为9.39%;退火温度为50.5~52.7 ℃时,随着温度的升高,条带质量变好,数量变多,退火温度为52.7 ℃时可获得多样性好的清晰条带。结论 获得ISSR-PCR反应体系为引物浓度0.4 μmol·L−1,2×Taq Master Mix添加量13 μL,DNA模板量30 ng,退火温度52.7 ℃,扩增循环数35循环,扩增获得的条带清晰、稳定,多样性好,该体系适于余甘子种质资源的遗传多样性和亲缘关系等分析研究。Abstract:Objective ISSR-PCR reaction system for genetic study on Phyllanthus emblica germplasms was optimized.Methods A mixed DNA template composed of genomic DNA of P. emblica germplasms came from Myanmar, India, Guangdong, Yunnan, and Fujian was obtained. The single factor test and response surface analysis were used to optimize the ISSR-PCR reaction conditions including primer concentration, amount of 2×Taq Master Mix, DNA template amount, and annealing temperature.Result The primer concentration and addition amount of 2×Taq Master Mix had a greater impact on the amplification than did the DNA template amount. A significant interaction between the primer concentration and DNA template amount was found. The optimized system with a primer concentration of 0.4 μmol·L−1, a 2×Taq Master Mix of 13 μL, and a DNA template concentration of 30 ng was established that achieved a low relative error of 9.39% in predicting the theoretical response. Within the annealing temperature between 50.5 ℃ and 52.7 ℃, increasing temperature improved the number and quality of bands. When the annealing temperature is 50.5-52.7 ℃, the number of strips increases, and the quality of the strips becomes better with the increase of temperature. The annealing temperature is 52.7 ℃ to obtain good diversity and clear bands.Conclusion The optimized ISSR-PCR reaction system was established applying a primer concentration of 0.4 μmol·L−1, a 2×Taq Master Mix of 13 L, a DNA template concentration of 30 ng at the annealing temperature of 52.7 ℃ for 35 amplification cycles. The methodology could be used to study the genetic diversity and relationship of P. emblica germplasms.
-
Keywords:
- Phyllanthus emblica /
- ISSR-PCR /
- response surface methodology /
- system optimization
-
0. 引言
【研究意义】 余甘子(Phyllanthus emblica L.),系大戟科(Euphorbiaceae)叶下珠属(Phyllanthus)多年生灌木或小乔木。余甘子的果实具有清热凉血、消食健胃、生津止咳的作用[1],历版的《中华人民共和国药典》均有收录[2]。余甘子主要分布于印度、泰国、马来西亚等热带和亚热带国家以及我国的海南、福建、广东、广西、云南等地[3],其中以中国和印度分布面积最大,产量最高[4]。我国的余甘子种质资源储有量十分丰富,90%以上仍处于野生或半野生状态,由于我国地域分布广大,余甘子命名繁杂,同名异种或同种异名的现象均很严重,对种质资源的收集保存和育种工作带来困难。开发简便、快速的分子标记技术,高效、及时地对余甘子种质资源进行系统精准评价分析,摸清遗传亲缘关系,对余甘子种质资源鉴定和选育种有重要意义。【前人研究进展】近年来,分子标记技术迅猛发展,由ZIETKIEWICZ等[5]1994年提出的简单重复序列区间(inter-simple sequence repeats,ISSR)标记成为分子辅助育种的重要手段之一,该技术具有操作简便、高效、成本低、无须明确任何靶标序列且稳定性好等优点[6],已被广泛地应用在橄榄[7],猕猴桃[8],山核桃[9]等多种野生果树的种质资源鉴定当中。但余甘子种质资源ISSR分子标记技术尚未被广泛应用,目前仅有刘晓生[10]、周春娟[11]分别对广东潮汕和粤东地区余甘子种质资源进行ISSR分子标记分析;邵雪花[12]对中国华南地区28份种质进行遗传多样性分析并构建遗传指纹图谱;李巧明等[13]对云南干热河谷地区的4个余甘子居群进行遗传多样性研究。以上研究均表明ISSR分子标记技术可有效运用于余甘子种质资源多样性分析。【本研究切入点】利用单因素试验并结合响应面分析法优化余甘子ISSR-PCR反应体系研究鲜有研究报道。【拟解决的关键问题】本研究通过应用单因素试验结合响应面分析法分析引物浓度、2×Taq Master Mix添加量、DNA模板量等反应条件对ISSR反应体系的影响及互相之间的交互作用,建立余甘子ISSR-PCR分子标记反应体系,为余甘子种质资源遗传多样性分析与亲缘关系研究提供研究基础。
1. 材料与方法
1.1 试验材料
2019年10月采集来源于缅甸、印度、广东、云南、福建的余甘子种质资源的健康嫩叶于−80 ℃保存备用。试验材料由国家热带植物种质资源库余甘子种质资源分库提供,位于福建省福州市晋安区新店镇埔垱村(119˚19′59ʺE,26˚7′48ʺN,海拔20 m)。品种(系)名及采集地见表1。
表 1 试验材料来源Table 1. Sources of testing materials编号
Code品种(系)名
Name来源
Source采样地
Sample Site1 缅甸实生 Myanmar seedling 缅甸 Myanmar 余甘子种质资源圃 Field genebank for Phyllanthus emblica 2 印度大果 India species 印度 India 余甘子种质资源圃 Field genebank for Phyllanthus emblica 3 甜种 Tianzhong 中国广东 Guangdong, China 余甘子种质资源圃 Field genebank for Phyllanthus emblica 4 盈玉 Yingyu 中国云南 Yunnan, China 余甘子种质资源圃 Field genebank for Phyllanthus emblica 5 福建本地种 Fujian native specie 中国福建 Fujian, China 余甘子种质资源圃 Field genebank for Phyllanthus emblica 主要试剂:2×Taq Master Mix(由Taq DNA聚合酶、dNTP混合物和MgCl2混合而成)购自天根生化科技(北京)有限公司,所用引物为UBC841(序列:GAG AGA GAG AGA GAG AYC)是UBC公布的ISSR通用引物,由北京六合华大基因科技有限公司合成。
主要仪器设备:离心机(SIGMA 1-14K)、电泳仪(BIO-RAD Power Pac Univer-sal)、电泳凝胶成像仪(BIO-RAD Gel DOCTMXR+)、超微量核酸测定仪(Thermo NANODROP ONE)、移液器(Eppendorf Research Plus)和PCR仪(BIO-RADT100TM)等。
1.2 试验方法
1.2.1 DNA提取
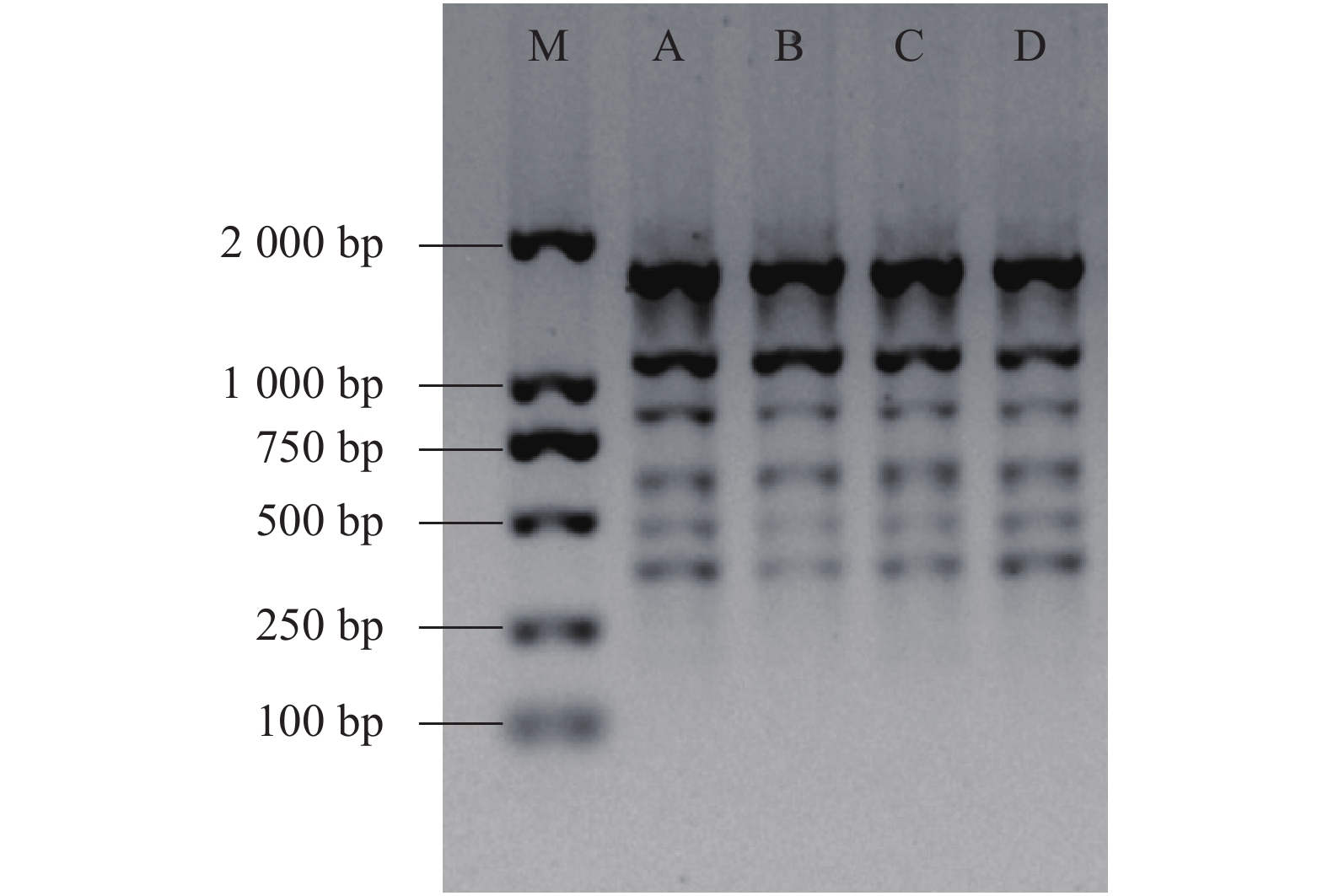
本研究选用不同地域来源的5份余甘子的DNA组成混合的DNA模板对ISSR反应体系进行优化,提取余甘子基因组DNA采用李金璐等[14]改良的CTAB法,选择DNA质量浓度为100~200 ng·μL−1,A260/A280比值为1.83~2.14,1%琼脂糖凝胶电泳条带完整性较好的DNA 样品于−80 ℃保存,进行后续试验。DNA模板各基因组DNA电泳效果见图1。
1.2.2 单因素及响应面优化试验设计
分别以引物浓度、2×Taq Master Mix添加量、DNA模板量3个单因素试验(表2)进行PCR扩增与凝胶电泳成像,明确体系反应条件的变化范围及各因素的适应值。在单因素结果基础上,根据 Box-Behnken Design的试验原理,每个自变量取3个水平,以−1、0、1进行编码(表3),以电泳图谱条带质量的评分为响应值,响应面试验设计见表4。电泳图谱条带质量的评分准则参考尚小红等[15]的主观分析法进行电泳扩增谱带分析,即根据电泳图谱中电泳条带数量、清晰度、重复性、亮度和背景干净程度分别对17个组合进行打分,1~4分(清晰条带少,亮度弱,杂带多,重复性差,背景模糊),5~8分(清晰条带多,但亮度较弱,有杂带,重复性好,背景较干净),9~12分(清晰条带多,亮度较强,无杂带,重复性好,背景清晰),13~16分(清晰条带多,亮度强,无杂带,重复性好,背景清晰)。
表 2 单因素试验设计Table 2. Simple factor experiment序号
Number编号
Code引物浓度
Primer
concentration/
(μmol·L−1)2×Taq Master Mix
添加量
2×Taq Master Mix
addition amount/μLDNA模板量
DNA template
amount/ng1 A 0.2 12 45 2 0.3 12 45 3 0.4 12 45 4 0.5 12 45 5 0.6 12 45 6 B 0.4 8 45 7 0.4 10 45 8 0.4 12 45 9 0.4 14 45 10 0.4 16 45 11 C 0.4 12 15 12 0.4 12 30 13 0.4 12 45 14 0.4 12 60 15 0.4 12 75 注:在整个反应过程中,随着比较因素梯度的设置变动,相应的调整ddH2O的量以保证反应体系为25 μL。
Note: During reaction process, amount of added ddH2O was continually adjusted to maintain volume of reaction system at 25 μL as gradient of comparison factor changed.表 3 响应面分析因子及水平表Table 3. Factors and levels of response surface design反应条件
Reation Condition编码
Code水平 Levels −1 0 1 引物浓度
Primer concentration/(μmol·L−1)X1 0.30 0.35 0.40 2×Taq Master Mix 添加量
2×Taq Master Mix addition amount/μLX2 12 13 14 DNA模板量
DNA template amount/ngX3 25 30 35 1.2.3 PCR扩增及电泳条件
扩增条件:反应体系为25 µL,反应程序为预变性94 ℃,5 min;变性94 ℃,45 s;退火温度53 ℃,45 s;延伸72 ℃,2 min;35个循环;终延伸72 ℃,6 min;最后于4 ℃保存。扩增完成后,取2 μL扩增产物染色点样于1%琼脂糖凝胶上,加0.5×TEB缓冲液,在120 V电场强度下电泳30 min,于凝胶成像仪上观察拍照。
1.2.4 退火温度的优化及体系适用性研究
以UBC 841为引物,利用最佳ISSR反应体系对PCR扩增程序中的退火温度进行单因素试验,退火温度使用自动梯度50.5、50.7、51.2、51.9、52.7、53.3、53.7、54.0 ℃共8个梯度。根据凝胶电泳图谱结果,选择条带清晰、重复性好、多态性好的温度为最佳退火温度。
1.3 统计分析
采用Excel 2016、Design Expert 8.0.6等软件进行统计分析,其中Design Expert 8.0.6进行响应面试验设计、数据分析和模型的建立。
2. 结果与分析
2.1 单因素对ISSR反应体系的影响
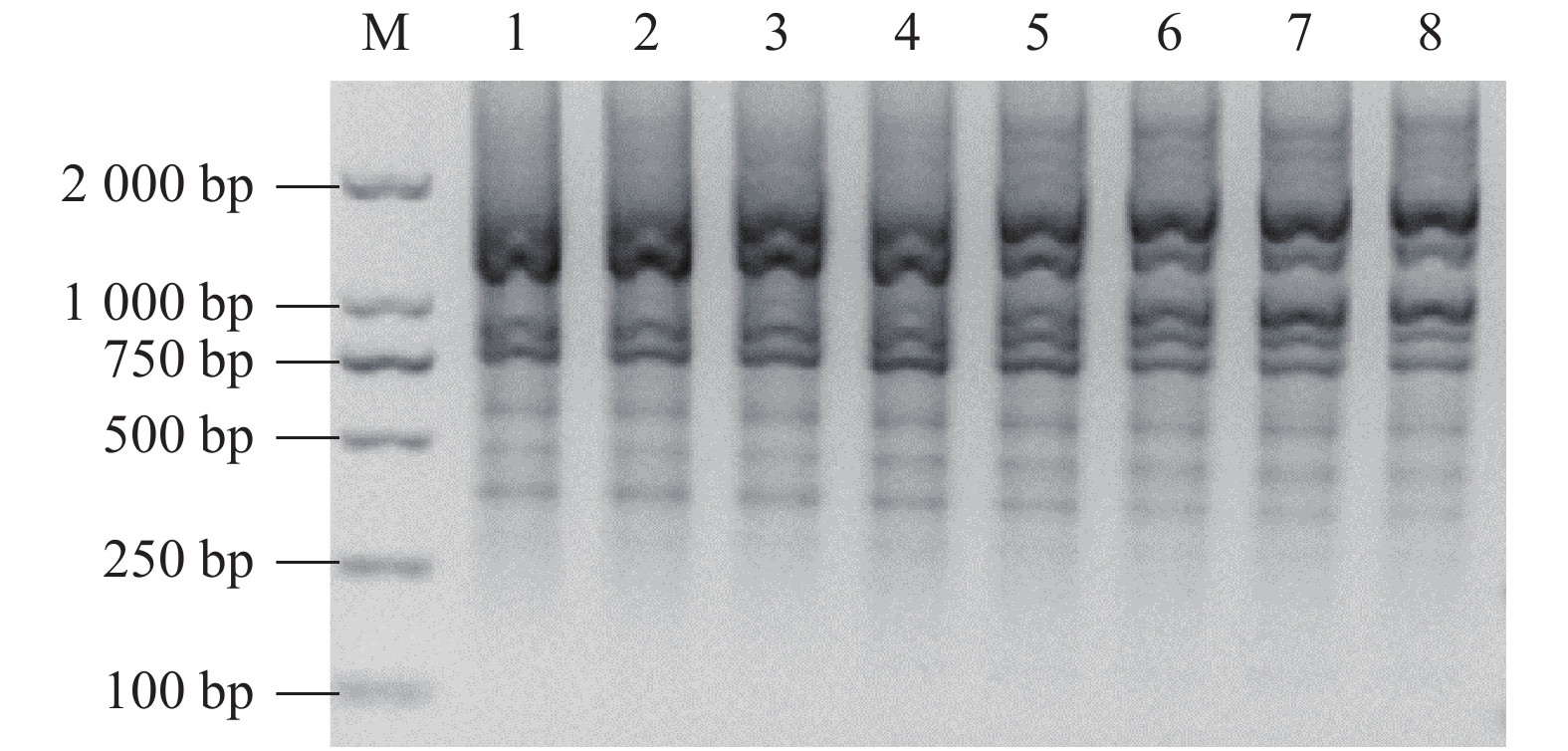
单因素试验的扩增产物经琼脂糖凝胶电泳结果如图2,可见,引物浓度与2×Taq Master Mix添加量对反应体系的影响较大。引物浓度偏高或偏低都会影响扩增结果,引物浓度过高会引起引物与DNA模板错配和非特异性产物扩增,增加产生二聚体的概率,引物浓度偏低则不易测出扩增位点,本研究引物浓度为0.30~0.40 μmol·L−1时电泳效果较好。2×Taq Master Mix含有Taq DNA聚合酶、dNTP混合物和Mg2+等反应原料,Taq DNA聚合酶直接影响扩增反应的成功与否,浓度过高容易产生非特异的PCR产物,dNTP是ISSR分子标记扩增的重要原料,浓度过高易错配,过低影响合成效率,2×Taq Master Mix添加量为12~14 μL为最佳。DNA模板量范围取决于物种基因组大小和DNA纯度,研究表明,DNA模板量对余甘子ISSR-PCR的影响较小,考虑电泳条带质量和数量,选择DNA模板量最佳为30 ng。
![]() 图 2 引物浓度(UBC 841)、2×Taq Master Mix添加量、DNA模板量对ISSR反应体系的影响注:M为DL15K DNA Maker;A、B、C分别表示引物浓度、2×Taq Master Mix添加量、DNA模板量单因素试验,1~15代表不同处理。Figure 2. Effect of primer concentration, 2×Taq Master Mix addition, and DNA template amount on ISSR reactionNote: M: DL15K DNA maker; A, B, and C: primer concentration, 2×Taq Master Mix addition, and DNA template amount, respectively, in single factor test; and 1-15: various treatments.
图 2 引物浓度(UBC 841)、2×Taq Master Mix添加量、DNA模板量对ISSR反应体系的影响注:M为DL15K DNA Maker;A、B、C分别表示引物浓度、2×Taq Master Mix添加量、DNA模板量单因素试验,1~15代表不同处理。Figure 2. Effect of primer concentration, 2×Taq Master Mix addition, and DNA template amount on ISSR reactionNote: M: DL15K DNA maker; A, B, and C: primer concentration, 2×Taq Master Mix addition, and DNA template amount, respectively, in single factor test; and 1-15: various treatments.2.2 响应面法优化ISSR反应体系
以电泳图谱(图3)中电泳条带数量、清晰度、重复性、亮度和背景干净程度的评分(5名科研工作者主观评分)为响应值(表4),采用多元回归拟合,获得引物浓度(X1),2×Taq Master Mix添加量(X2),DNA模板量(X3)的二次多项回归模型为F=11.92−0.4375X1−0.55X2−1.7375X3−0.75X1X2−1.575X1X3−0.55X2X3−4.2975X12−3.5225X22−2.1975X32。检验方程的有效性,对数学模型进行方差分析电泳图评分为响应值时,该二次方程模型有统计学意义(p=0.012 6<0.05),说明该模型对本试验有较好拟合;回归方程失拟性检验无统计学意义(p=0.757 1),表明未知因素对试验结果干扰很小,模型能较好地反应真实的试验值,可用于响应值的分析和预测,所得的回归方程模型能较好地预测电泳图谱得分随各参数的变化规律。试验所选三因子X1、X2和X3中X2达到显著影响(p<0.05),X1、X3因子二次项均达到极显著影响(p<0.001)表明单因素X2对响应结果影响较大,且X1与X3因子有极显著的交互影响。
表 4 响应面分析方案及试验结果Table 4. Design and results on factors and levels of response surface test序号
Order numberX1 X2 X3 评分
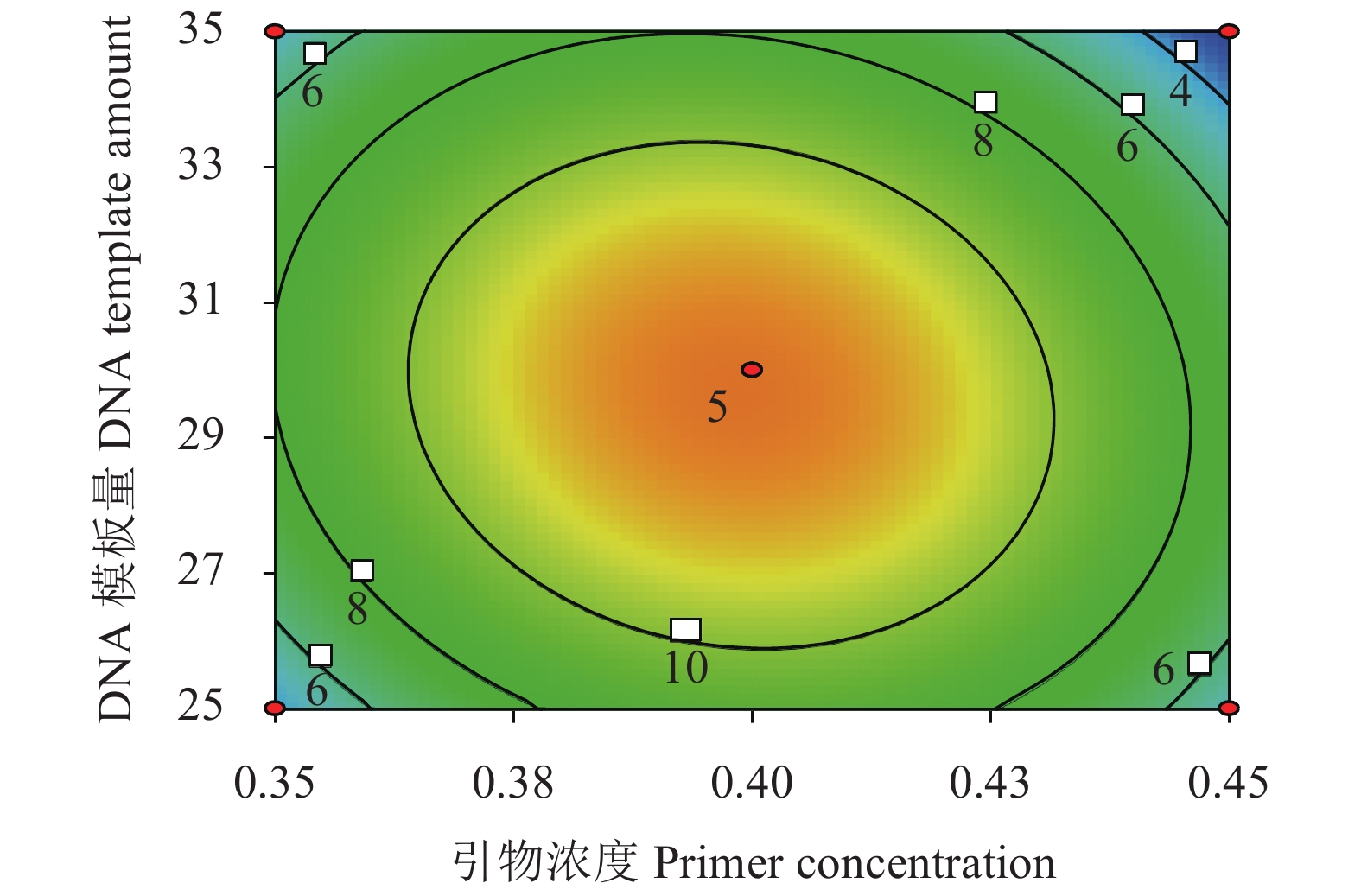
Score1 0 0 0 14.2±1.41 2 0 1 −1 4.25±1.26 3 1 0 1 2.12±1.26 4 1 −1 0 9.25±0.5 5 −1 −1 0 10.25±0.82 6 1 0 −1 6.75±0.96 7 0 −1 1 6.38±1.60 8 1 1 0 9.38±0.95 9 0 −1 −1 8.13±0.85 10 0 0 0 13.55±2.46 11 0 1 1 7.38±1.38 12 0 0 0 10.00±1.83 13 1 0 1 5.13±0.82 14 0 1 0 8.75±0.96 15 −1 0 −1 2.25±0.50 16 −1 0 1 7.50±1.29 17 0 0 0 14.75±5.19 根据回归拟合方程得出各试验因子引物浓度(X1)、2×Taq Master Mix添加量(X2)、DNA模板量(X3)两两因素交互作用的等高线图(图4),等高线图可直观反映出2个变量间交互作用的显著程度,其中圆形表示两两因素交互作用不显著,而椭圆形表示两两因素交互作用显著[16];等高线图由蓝到红的变化越快,沿自变量方向高度差越大[17],引物浓度(X1)和DNA模板量(X3)之间交互作用显著。
根据Design Expert 8.0.6 软件分析出的若干解决方案与其相应预测得分值,考虑到保证高响应值的同时节约成本,对响应面优化结果进行最优分析验证,得到ISSR反应体系的实际值(图5),该值与理论预测值进行比较计算相对误差,结果见表 5。结果表明,最优实际解决方案引物浓度为0.40 μmol·L−1,2×Taq Master Mix添加量为13.0 μL,DNA模板量30 ng,该条件与理论预测响应值13.130 4的相对误差仅为9.39%,说明该模型具有好的分析能力,可为实际操作提供良好的指导。
表 5 验证试验与结果Table 5. Validation test results序号
Number理论解决方案
Theoretical solution实际解决方案
Practical solution理论评分
Theoretical score实际评分
Actual score相对误差
Relative error/%1 X1:0.36 μmol·L−1,X2:13.46 μL,X3:29.41 ng X1:0.35 μmol·L−1,X2:13.5 μL,X3:30 ng 13.1149 11.4±0.55 9.58 2 X1:0.37 μmol·L−1,X2:13.09 μL,X3:29.06 ng X1:0.40 μmol·L−1,X2:13.0 μL,X3:30 ng 13.1304 12.5±0.79 9.39 2.3 退火温度对ISSR反应体系的影响
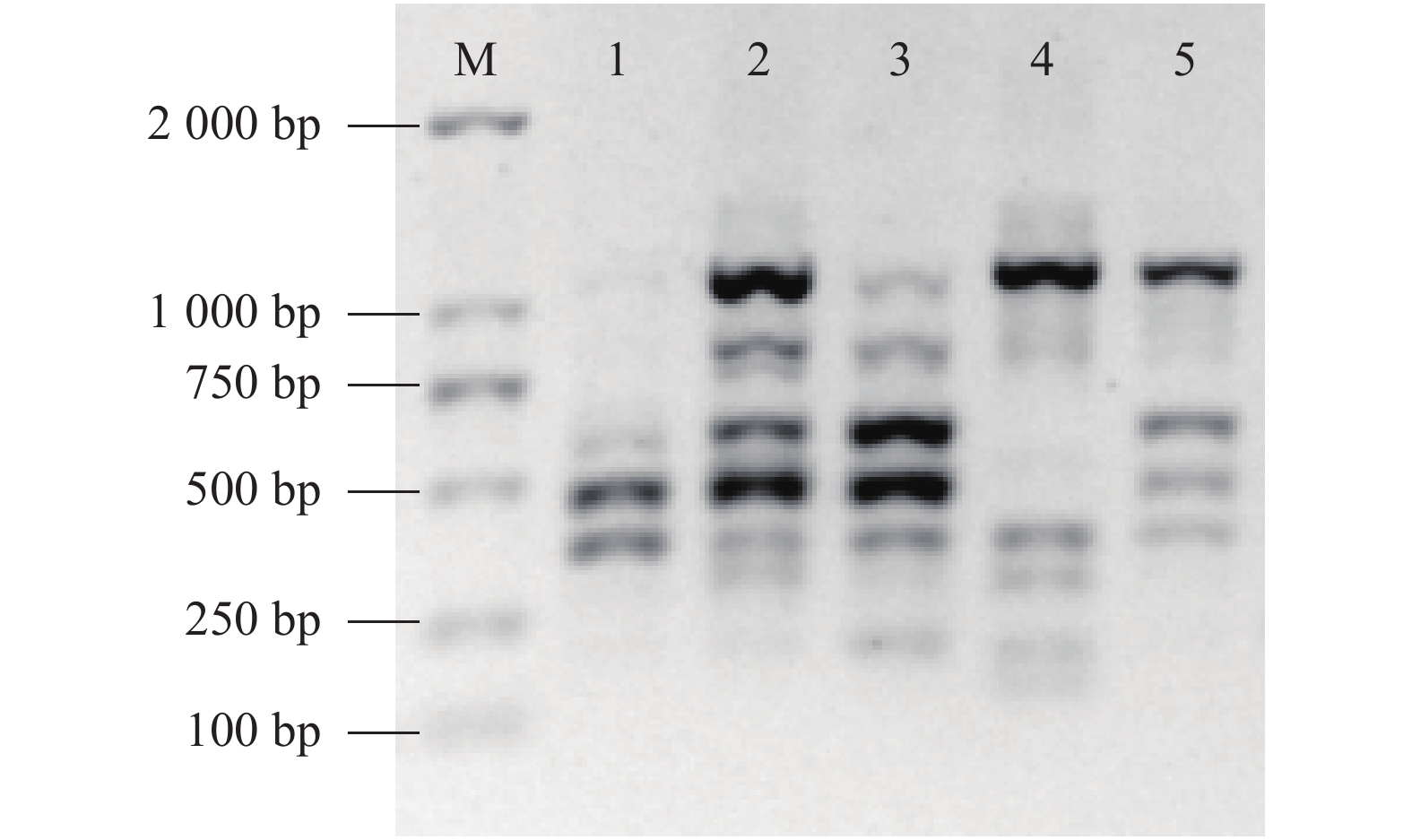
退火温度影响ISSR图谱的稳定性,较低的退火温度可保证引物与模板结合的稳定性,但较易产生错误的扩增。由电泳图谱(图6)可知,35循环数下,50.5~54.0 ℃随着退火温度的升高谱带质量与条带数有较明显的变化,退火温度为50.5~52.7 ℃时,随着温度的升高条带数呈增加趋势,条带质量变好,52.7~54.0 ℃时,部分条带模糊,缺失。选择较高的退火温度可减少引物和模板间非特异性的结合,提高扩增产物的特异性,综合谱带质量和条带数,退火温度为52.7 ℃时最佳,可获得较好的扩增效果。
![]() 图 6 退火温度对ISSR反应体系的影响注:M为DL2K DNA Marker;电泳谱1~8依次表示50.5 、50.7、51.2 、51.9 、52.7 、53.3、53.7 和54.0 ℃退火温度下扩增效果。Figure 6. Effect of annealing temperature on ISSR assayNote: M: DL2K DNA marker; Electrophoresis 1-8: at ISSR annealing temperatures of 50.5 , 50.7 , 51.2 , 51.9, 52.7, 53.3 , 53.7 and 54.0 ℃, respectively.
图 6 退火温度对ISSR反应体系的影响注:M为DL2K DNA Marker;电泳谱1~8依次表示50.5 、50.7、51.2 、51.9 、52.7 、53.3、53.7 和54.0 ℃退火温度下扩增效果。Figure 6. Effect of annealing temperature on ISSR assayNote: M: DL2K DNA marker; Electrophoresis 1-8: at ISSR annealing temperatures of 50.5 , 50.7 , 51.2 , 51.9, 52.7, 53.3 , 53.7 and 54.0 ℃, respectively.通过优化得到ISSR-PCR反应的体系为引物浓度为0.4 μmol·L−1,2×Taq Master Mix添加量为13 μL,DNA模板量30 ng,退火温度为52.7 ℃,循环次数35次;将该体系应用于不同余甘子种质资源效果见图7,引物UBC841在该体系下可扩增出条带清晰和多态性良好的电泳图谱,表明该体系适宜应用于余甘子种质资源的遗传多样性和亲缘关系研究。
3. 讨论与结论
ISSR是在SSR的基础上发展起来的一种新型的分子标记,目前,ISSR已广泛用于植物品种鉴定、遗传图谱、遗传多样性及分子生态学研究[18, 19]。ISSR分子标记技术基于PCR反应,其扩增谱带受反应条件和扩增程序变化以及物种不同的影响,采用不同的反应体系组合和扩增程序电泳结果差异较大,因此,开发ISSR分子标记对其反应体系和扩增程序优化显得必不可少。
本研究综合运用单因素试验与响应面分析法,首先确定了各因素的有效范围,再对各因素组合优化,从而确立普遍适用于余甘子种质资源的ISSR-PCR反应体系。本研究PCR扩增采用2×Taq Master Mix试剂,该试剂将多种扩增原料混合,研究结果虽未能直观体现Taq DNA聚合酶、dNTPs和Mg2+ 等原料对余甘子ISSR-PCR反应体系的影响[20],但可大大提高工作效率,达到了节省物料、简便、快速的目的,可在ISSR分子标记研究中加以推广应用。本研究结果表明引物浓度对余甘子ISSR反应体系影响较大,DNA模板量与引物浓度有显著的交互作用,与前人研究较一致[21, 22]。应用响应面法对影响ISSR反应体系的主要因素进行筛选,建立余甘子ISSR反应体系模型F=11.92−0.437 5X1−0.55X2−1.737 5X3−0.75X1X2−1.575X1X3−0.55X2X3−4.297 5X12−3.522 5X22−2.197 5X32,可较快地得到最优组合,避免了单因素试验结果的不足。
本研究确立余甘子ISSR-PCR反应体系:引物浓度为0.4 μmol·L−1,2×Taq Master Mix添加量为13 μL,DNA模板浓度30 ng,扩增循环数为35循环,退火温度52.7 ℃;扩增获得的条带清晰、稳定,多样性好,可应用于余甘子种质资源的遗传多样性分析和亲缘关系研究。
-
图 2 引物浓度(UBC 841)、2×Taq Master Mix添加量、DNA模板量对ISSR反应体系的影响
注:M为DL15K DNA Maker;A、B、C分别表示引物浓度、2×Taq Master Mix添加量、DNA模板量单因素试验,1~15代表不同处理。
Figure 2. Effect of primer concentration, 2×Taq Master Mix addition, and DNA template amount on ISSR reaction
Note: M: DL15K DNA maker; A, B, and C: primer concentration, 2×Taq Master Mix addition, and DNA template amount, respectively, in single factor test; and 1-15: various treatments.
图 6 退火温度对ISSR反应体系的影响
注:M为DL2K DNA Marker;电泳谱1~8依次表示50.5 、50.7、51.2 、51.9 、52.7 、53.3、53.7 和54.0 ℃退火温度下扩增效果。
Figure 6. Effect of annealing temperature on ISSR assay
Note: M: DL2K DNA marker; Electrophoresis 1-8: at ISSR annealing temperatures of 50.5 , 50.7 , 51.2 , 51.9, 52.7, 53.3 , 53.7 and 54.0 ℃, respectively.
表 1 试验材料来源
Table 1 Sources of testing materials
编号
Code品种(系)名
Name来源
Source采样地
Sample Site1 缅甸实生 Myanmar seedling 缅甸 Myanmar 余甘子种质资源圃 Field genebank for Phyllanthus emblica 2 印度大果 India species 印度 India 余甘子种质资源圃 Field genebank for Phyllanthus emblica 3 甜种 Tianzhong 中国广东 Guangdong, China 余甘子种质资源圃 Field genebank for Phyllanthus emblica 4 盈玉 Yingyu 中国云南 Yunnan, China 余甘子种质资源圃 Field genebank for Phyllanthus emblica 5 福建本地种 Fujian native specie 中国福建 Fujian, China 余甘子种质资源圃 Field genebank for Phyllanthus emblica 表 2 单因素试验设计
Table 2 Simple factor experiment
序号
Number编号
Code引物浓度
Primer
concentration/
(μmol·L−1)2×Taq Master Mix
添加量
2×Taq Master Mix
addition amount/μLDNA模板量
DNA template
amount/ng1 A 0.2 12 45 2 0.3 12 45 3 0.4 12 45 4 0.5 12 45 5 0.6 12 45 6 B 0.4 8 45 7 0.4 10 45 8 0.4 12 45 9 0.4 14 45 10 0.4 16 45 11 C 0.4 12 15 12 0.4 12 30 13 0.4 12 45 14 0.4 12 60 15 0.4 12 75 注:在整个反应过程中,随着比较因素梯度的设置变动,相应的调整ddH2O的量以保证反应体系为25 μL。
Note: During reaction process, amount of added ddH2O was continually adjusted to maintain volume of reaction system at 25 μL as gradient of comparison factor changed.表 3 响应面分析因子及水平表
Table 3 Factors and levels of response surface design
反应条件
Reation Condition编码
Code水平 Levels −1 0 1 引物浓度
Primer concentration/(μmol·L−1)X1 0.30 0.35 0.40 2×Taq Master Mix 添加量
2×Taq Master Mix addition amount/μLX2 12 13 14 DNA模板量
DNA template amount/ngX3 25 30 35 表 4 响应面分析方案及试验结果
Table 4 Design and results on factors and levels of response surface test
序号
Order numberX1 X2 X3 评分
Score1 0 0 0 14.2±1.41 2 0 1 −1 4.25±1.26 3 1 0 1 2.12±1.26 4 1 −1 0 9.25±0.5 5 −1 −1 0 10.25±0.82 6 1 0 −1 6.75±0.96 7 0 −1 1 6.38±1.60 8 1 1 0 9.38±0.95 9 0 −1 −1 8.13±0.85 10 0 0 0 13.55±2.46 11 0 1 1 7.38±1.38 12 0 0 0 10.00±1.83 13 1 0 1 5.13±0.82 14 0 1 0 8.75±0.96 15 −1 0 −1 2.25±0.50 16 −1 0 1 7.50±1.29 17 0 0 0 14.75±5.19 表 5 验证试验与结果
Table 5 Validation test results
序号
Number理论解决方案
Theoretical solution实际解决方案
Practical solution理论评分
Theoretical score实际评分
Actual score相对误差
Relative error/%1 X1:0.36 μmol·L−1,X2:13.46 μL,X3:29.41 ng X1:0.35 μmol·L−1,X2:13.5 μL,X3:30 ng 13.1149 11.4±0.55 9.58 2 X1:0.37 μmol·L−1,X2:13.09 μL,X3:29.06 ng X1:0.40 μmol·L−1,X2:13.0 μL,X3:30 ng 13.1304 12.5±0.79 9.39 -
[1] 潘慧清, 朱平, 魏学明, 等. 藏药余甘子研究概况 [J]. 甘肃中医药大学学报, 2019, 36(2):84−88. PAN H Q, ZHU P, WEI X M, et al. On Tibetan medicine Yuganzi (Phylianthi fructus) [J]. Journal of Gansu University of Chinese Medicine, 2019, 36(2): 84−88.(in Chinese)
[2] 国家药典委员会. 中华人民共和国药典: 一部[M]. 北京: 中国医药科技出版社, 2020: 186-187. [3] 赵谋明, 刘晓丽, 崔春, 等. 余甘子多酚响应面法优化提取及其抗氧化活性研究 [J]. 食品工业科技, 2007, 28(6):117−120. DOI: 10.3969/j.issn.1002-0306.2007.06.034 ZHAO M M, LIU X L, CUI C, et al. Study on the optimizing extraction processing of polyphenol from Phyllanthus emblica L. fruit by method of response surface analysis and its antioxidant activity [J]. Science and Technology of Food Industry, 2007, 28(6): 117−120.(in Chinese) DOI: 10.3969/j.issn.1002-0306.2007.06.034
[4] GANTAIT S, MAHANTA M, BERA S, et al. Advances in biotechnology of Emblica officinalis Gaertn. syn. Phyllanthus emblica L. : A nutraceuticals-rich fruit tree with multifaceted ethnomedicinal uses [J]. 3 Biotech, 2021, 11(2): 1−25.
[5] ZIETKIEWICZ E, RAFALSKI A, LABUDA D. Genome fingerprinting by simple sequence repeat (SSR)-anchored polymerase chain reaction amplification [J]. Genomics, 1994, 20(2): 176−183. DOI: 10.1006/geno.1994.1151
[6] SARWAT M, DAS S, SRIVASTAVA P S. Analysis of genetic diversity through AFLP, SAMPL, ISSR and RAPD markers in Tribulus terrestris, a medicinal herb [J]. Plant Cell Reports, 2008, 27(3): 519−528. DOI: 10.1007/s00299-007-0478-5
[7] 杨培奎, 郑道序, 马瑞君, 等. 潮汕橄榄地方品种(系)遗传多样性的ISSR分析 [J]. 广东农业科学, 2013, 40(23):129−132. DOI: 10.3969/j.issn.1004-874X.2013.23.031 YANG P K, ZHENG D X, MA R J, et al. Genetic diversity analysis of Canarium album L. landrances in Chaoshan area by ISSR [J]. Guangdong Agricultural Sciences, 2013, 40(23): 129−132.(in Chinese) DOI: 10.3969/j.issn.1004-874X.2013.23.031
[8] 张安世, 韩臣鹏, 齐秀娟, 等. 基于ISSR标记的猕猴桃品种遗传多样性分析及指纹图谱构建 [J]. 植物资源与环境学报, 2017, 26(3):19−26. DOI: 10.3969/j.issn.1674-7895.2017.03.03 ZHANG A S, HAN C P, QI X J, et al. Genetic diversity analysis and fingerprinting construction of cultivars of Actinidia spp. based on ISSR marker [J]. Journal of Plant Resources and Environment, 2017, 26(3): 19−26.(in Chinese) DOI: 10.3969/j.issn.1674-7895.2017.03.03
[9] 李国田, 张美勇, 相昆, 等. 基于ISSR标记的16个核桃品种遗传多样性分析及分子身份构建 [J]. 核农学报, 2015, 29(10):1884−1892. DOI: 10.11869/j.issn.100-8551.2015.10.1884 LI G T, ZHANG M Y, XIANG K, et al. Analysis of genetic diversity and establishment of molecular ID for 16 walnut varieties based on ISSR markers [J]. Journal of Nuclear Agricultural Sciences, 2015, 29(10): 1884−1892.(in Chinese) DOI: 10.11869/j.issn.100-8551.2015.10.1884
[10] 刘晓生, 郑道序, 周春娟, 等. 潮汕余甘子种质资源遗传多样性与亲缘关系的ISSR分析 [J]. 中国南方果树, 2014, 43(1):18−22. LIU X S, ZHENG D X, ZHOU C J, et al. Analysis of genetic diversity and genetic relationship of Phyllanthus emblica L. Germplasm in Chaoshan area with ISSR [J]. South China Fruits, 2014, 43(1): 18−22.(in Chinese)
[11] 周春娟, 詹潮安, 刘晓生, 等. 粤东余甘子种质资源遗传多样性与亲缘关系的ISSR分析[C]//广东省植物学会年会2012年年会论文集. 2012: 16-16. ZHOU C J, ZHAN C A, LIU X S, et al. ISSR Analysis of genetic diversity and genetic relationship of Phyllanthus emblica germplasm resources in eastern Guangdong[C] //Annual meeting of Guangdong Botany Society. 2012: 16-16. (in Chinese)
[12] 邵雪花, 刘牛, 赖多, 等. 28份余甘子品种遗传多样性的ISSR分析及指纹图谱构建 [J]. 西北农林科技大学学报(自然科学版), 2020, 48(8):129−136. SHAO X H, LIU N, LAI D, et al. Genetic diversity analysis and DNA fingerprint mapping of 28 varieties of Phyllanthus emblica L. based on ISSR molecular marker [J]. Journal of Northwest A & F University (Natural Science Edition), 2020, 48(8): 129−136.(in Chinese)
[13] 李巧明, 赵建立. 云南干热河谷地区余甘子居群的遗传多样性研究 [J]. 生物多样性, 2007, 15(1):84−91. DOI: 10.3321/j.issn:1005-0094.2007.01.009 LI Q M, ZHAO J L. Genetic diversity of Phyllanthus emblica populations in dry-hot valleys in Yunnan [J]. Biodiversity Science, 2007, 15(1): 84−91.(in Chinese) DOI: 10.3321/j.issn:1005-0094.2007.01.009
[14] 李金璐, 王硕, 于婧, 等. 一种改良的植物DNA提取方法 [J]. 植物学报, 2013, 48(1):72−78. DOI: 10.3724/SP.J.1259.2013.00072 LI J L, WANG S, YU J, et al. A modified CTAB protocol for plant DNA extraction [J]. Chinese Bulletin of Botany, 2013, 48(1): 72−78.(in Chinese) DOI: 10.3724/SP.J.1259.2013.00072
[15] 尚小红, 严华兵, 曹升, 等. 葛根SCoT-PCR反应体系优化及引物筛选 [J]. 南方农业学报, 2018, 49(1):1−7. DOI: 10.3969/j.issn.2095-1191.2018.01.01 SHANG X H, YAN H B, CAO S, et al. Optimization of SCoT-PCR reaction system and primer selection for Pueraria DC [J]. Journal of Southern Agriculture, 2018, 49(1): 1−7.(in Chinese) DOI: 10.3969/j.issn.2095-1191.2018.01.01
[16] 阚琦缤, 刘瑞雪, 王晓娅, 等. 响应面优化刺五加总黄酮提取工艺及体外抗氧化研究 [J]. 福建农业学报, 2021, 36(3):358−368. KAN Q B, LIU R X, WANG X Y, et al. Process optimization and in vitro antioxidant activity of flavonoids extracted from Acanthopanax senticosus [J]. Fujian Journal of Agricultural Sciences, 2021, 36(3): 358−368.(in Chinese)
[17] 郑良, 李越凡, 赵强, 等. 基于响应面分析的聚甲基丙烯酸甲酯表面微观生物污损超声防除研究 [J]. 表面技术, 2021, 50(4):319−327. ZHENG L, LI Y F, ZHAO Q, et al. Research on removal of microfouling on polymethyl methacrylate surface by ultrasonic antifouling technology based on response surface analysis [J]. Surface Technology, 2021, 50(4): 319−327.(in Chinese)
[18] YEH F C. Population genetic analysis of codominant and dominant markers and quantitative traits [J]. Belgian Journal of Botany, 1997: 129.
[19] 王建波. ISSR分子标记及其在植物遗传学研究中的应用 [J]. 遗传, 2002, 24(5):613−616. DOI: 10.3321/j.issn:0253-9772.2002.05.022 WANG J B. ISSR markers and their applications in plant genetics [J]. Hereditas(Beijing), 2002, 24(5): 613−616.(in Chinese) DOI: 10.3321/j.issn:0253-9772.2002.05.022
[20] 黄晓慧, 巫伟峰, 陈春, 等. 中国兰ISSR-PCR反应体系优化及引物筛选 [J]. 南方农业学报, 2018, 49(7):1282−1288. DOI: 10.3969/j.issn.2095-1191.2018.07.04 HUANG X H, WU W F, CHEN C, et al. Optimization and primer screening of ISSR-PCR reaction system for Chinese Orchids [J]. Journal of Southern Agriculture, 2018, 49(7): 1282−1288.(in Chinese) DOI: 10.3969/j.issn.2095-1191.2018.07.04
[21] 刘凤书, 侯开卫, 李绍家, 等. 余甘子的保健价值及开发利用前景 [J]. 自然资源学报, 1993, 8(4):299−306. DOI: 10.3321/j.issn:1000-3037.1993.04.002 LIU F S, HOU K W, LI S J, et al. The health-protecting value of Phyllanthus emblica L. and its prospects for exploitation and utilization [J]. Journal of Natural Resources, 1993, 8(4): 299−306.(in Chinese) DOI: 10.3321/j.issn:1000-3037.1993.04.002
[22] 钟凤林, 王江波, 潘东明, 等. 余甘子ISSR反应体系的优化 [J]. 生物技术通报, 2008(3):166−169. ZHONG F L, WANG J B, PAN D M, et al. Optimization of ISSR reaction system in Phyllanthus emblica L [J]. Biotechnology Bulletin, 2008(3): 166−169.(in Chinese)
-
期刊类型引用(0)
其他类型引用(1)




 下载:
下载: