Cloning and Localization of Gonadal Aromatase Gene in Silurus lanzhouensis
-
摘要:目的 性腺型芳香化酶基因cyp19a1a在硬骨鱼类性腺发育和性别决定过程中具有重要作用,克隆分析该基因在生长和体型性状具有性别二态性兰州鲇(Silurus lanzhouensis)不同组织的表达与定位,并对已获得YY超雄个体的雌雄同体亲本进行不同组织表达验证分析,为加快培育全雄兰州鲇良种新品种提供理论依据和技术支撑。方法 采用同源克隆和cDNA末端快速扩增(RACE)法获得兰州鲇cyp19a1a全长cDNA序列,采用实时荧光定量PCR(qRT-PCR)分别检测该基因在3月龄、1龄和3龄兰州鲇正常雌雄鱼和3龄雌雄同体鱼的不同组织表达,并采用免疫组织化学法(IHC)分别检测该基因在3月龄、1龄和3龄正常雌性鱼性腺组织的表达与定位。结果 cyp19a1a cDNA序列全长为2168 bp,其中5′ 非编码区(Untranslated region,UTR)53 bp,开放阅读框(OFR)1707 bp,3′ UTR 408 bp,编码568个氨基酸残基,具有芳香化酶氨基酸序列的保守功能区。兰州鲇cyp19a1a mRNA主要在性腺表达,3龄雌雄同体表达特征与3龄正常繁育个体相同,且卵巢表达量显著高于精巢(P < 0.05);正常繁育养殖个体表达量随性腺发育而显著增加。IHC结果显示,不同时期卵巢组织中,卵原细胞仅在3月龄和1龄分布且没有发现阳性信号,卵母细胞在不同时期均有分布且信号随着卵母细胞发育而逐渐增强,其中3月龄至1龄主要分布于胞质,3龄时主要分布于胞质、滤泡膜以及放射膜和鞘膜细胞;不同时期精巢组织中仅在间质细胞有微弱表达。结论 cyp19a1a基因在兰州鲇卵巢发育和卵细胞生长发育过程中都具有重要作用,对精巢发育和维持以及精子发生具有一定的促进作用,在雌雄同体兰州鲇性腺中的作用和兰州鲇正常繁育个体一致。本研究为鱼类性别决定与分化以及性腺发育调控机制提供了重要的理论依据。Abstract:Objective Expression and location of cyp19a1a important in the gonadal development and sex determination of bony fishes were studied to pave the way for breeding all-male Silurus lanzhouensis.Method The gene in the sexual organs of S. lanzhouensis were cloned, and expression of hermaphroditic parents of YY super-male individuals verified. The full-length cDNA sequence of cyp19a1a was obtained by homologous cloning and rapid amplification of cDNA ends (RACE). Expressions of the gene in 3-month-old, 1-year-old, and 3-year-old normal female and male as well as 3-year-old hermaphroditic fish were determined by real-time fluorescence quantitative PCR (qRT-PCR). Expression and localization of the gene in tissues of 3-month-old, 1-year-old, and 3-year-old normal female fish were determined using immunohistochemistry (IHC).Result The full length of cyp19a1a cDNA was 2168 bp, which included 5' untranslated region (UTR) of 53 bp, open reading frame (OFR) of 1707 bp, and 3' UTR of 408 bp, encoding 568 amino acid residues with a conserved functional region of aromatase. Cyp19a1a mRNA was mainly expressed in the gonads. The expressions in 3-year-old hermaphroditic and normal individuals were not significantly different (P>0.05), but significantly higher in ovary than in testis, and significantly intensified along with the development of gonad in the bred normal fish. The IHC showed the oogonia distributed only in the ovaries of 3-month and 1-year-old fish at different stages with no positive signals. Whereas the oocytes were found during all development periods with a gradually increasing signal. They existed mainly in the cytoplasm from 3-month to 1-year-old, but in the 3-year-old fish in the follicular membrane, radiation membrane, and sheath cells, in addition to cytoplasm. On the other hand, cyp19a1a was only weakly expressed in the interstitial cells of the testis at different developmental stages of a fish.Conclusion There was a significant role cyp19a1a played in the ovarian development, oocyte growth, and oogenesis. It involved in the testicular development and maintenance and spermatogenesis of normal or hermaphroditic S. lanzhouensis identically.
-
Keywords:
- Silurus lanzhouensis /
- hermaphroditism /
- cyp19a1a gene /
- gene cloning /
- gonadal development /
- mRNA expression /
- cell localization
-
辣木Moringa oleifera Lam.原产印度,为辣木科辣木属植物,是一种具有较高经济价值的多用途树种,目前世界各地均有引种栽培[1-2]。辣木的叶片、花、嫩荚和种子等均可食用,且具有较高的食用价值[3-4]。辣木叶片富含蛋白质、矿物质、β-胡萝卜素和维生素等,是天然抗氧化剂的良好来源[5]。辣木种子富含不饱和脂肪酸,可作为优质食用油[6]。辣木还具有重要的药用价值,对高血压、糖尿病、失眠和贫血等均具有很好的治疗作用[5, 7]。此外,辣木还可作为优质蛋白质饲料[8],具有广阔的发展前景。然而,生产上存在辣木种源谱系不清、实生苗个体差异大等问题[9]。因此,建立和完善辣木品种资源分类和鉴定体系,对辣木产业的发展具有重要的意义。
分子标记技术是有效的种质资源鉴定途径[10]。分子标记在辣木种质资源研究中的成功应用已有报道[9, 11-14]。SSR(Simple sequence repeats)标记集共显性、多态性高、重复性好、易操作和检测等诸多优点于一身[11],广泛地用于植物遗传多样性分析、品种鉴定和亲缘关系研究等领域[15-18]。EST-SSR可直接反映植物功能基因的多样性,且通用性好[19]。本研究以辣木叶片RNA-Seq(RNA Sequencing)数据为基础,分析辣木转录组SSR位点信息,为SSR引物开发以及辣木种质资源多样性、品种鉴定及亲缘关系研究等奠定基础。
1. 材料与方法
1.1 转录组数据
辣木转录组数据为本课题组于2016年采用Illumina高通量测序获得的辣木不同阶段叶片(展叶期后0、10、20、30 d)的转录组数据。测序时,分别提取不同阶段叶片的RNA并等量混合用于文库构建。委托北京百迈客生物科技有限公司采用Illumina HiSeq 4000进行测序,原始数据已提交至NCBI SRA数据库(编号SRR5765086)。测序数据经过滤后,采用Trinity进行序列组装。
1.2 辣木转录组SSR位点鉴别与分析
提取辣木叶转录组中序列长度大于1 kb的Unigene,并采用MISA(MIcroSAtellite identification tool)软件(http://pgrc.ipk-gatersleben.de/misa/misa.html)进行SSR(包括单碱基重复SSR、双碱基重复SSR、三碱基重复SSR、四碱基重复SSR、五碱基重复SSR、六碱基重复SSR和混合SSR)位点的搜索。SSR位点搜索条件为单核苷酸重复不低于10次,二核苷酸重复不低于6次,三核苷酸、四核苷酸、五核苷酸和六核苷酸重复不低于5次,2个SSR位点间的距离不超过100 bp即认定为复合SSR。
2. 结果与分析
2.1 辣木转录组中SSR的分布及结构特点
辣木叶片转录组共组装得到75 452条Unigene,其中有15 824条Unigene的长度大于1 kb,大于1 kb的Unigene序列总长度约为36 676 kb。采用MISA软件对这些序列进行SSR搜索,结果表明含有SSR位点的Unigene共有6 003条,其中1 695条Unigene(28.24%)含有2个或2个以上的SSR位点,432条Unigene含有复合型SSR。共检测到8 272个SSR位点,SSR发生频率为52.28%(检出的SSR位点总数8 272/大于1 kb的Unigene数15 824),平均每4.43 kb含有1个SSR位点,其中复合SSR有449个(7.22%)。如表 1所示,有单核苷酸至六核苷酸6种重复类型的SSR位点存在。其中单核苷酸重复出现频率最高,占总SSR位点的55.69%,其次为二核苷酸和三核苷酸重复,分别占总SSR的32.34%和11.24%;四、五和六核苷酸重复出现频率均较低,分别为总SSR的0.54%、0.06%和0.12%。
表 1 辣木转录组SSR不同基序长度和重复次数的数量分布Table 1. Distribution of SSRs with different motif types and repeating numbers in transcriptome of M. oleifera基序长度/bp 重复次数 总计 比例/% 5 6 7 8 9 10~20 >20 1 0 0 0 0 0 4522 85 4607 55.69 2 0 769 501 452 429 524 0 2675 32.34 3 579 217 108 21 3 2 0 930 11.24 4 37 6 2 0 0 0 0 45 0.54 5 4 1 0 0 0 0 0 5 0.06 6 1 5 3 0 0 1 0 10 0.12 总计 621 998 614 473 432 5049 85 8272 比例/% 7.51 12.06 7.42 5.72 5.22 61.04 1.03 辣木转录组SSR重复单元的重复次数分布在5~24次,其中10~20次重复的SSR最多,共有5 049个,占总数的61.04%。;5~9次重复的SSR共有3 138个,占总数的37.94%。20次重复以上的SSR仅85个,占总数的1.03%。辣木转录组SSR的长度从10~279 bp不等,其中12~19 bp的SSR最多,占总数的59.58%(4 637个),其次为小于12 bp的SSR(1 940个,占24.93%)和大于19 bp的SSR有(1 208个,占15.53%)。大于100 bp的SSR共有89个。
2.2 辣木转录组SSR基序重复类型和频率特征
从核苷酸基序类型来看,辣木转录组中的8 272个SSR位点共包含114种重复单元,一、二、三、四、五、六核苷酸重复各有4、12、54、29、5、10种。从分布频率(表 2)来看,出现最多的基元是A/T(4 452个,53.82%),其次是AG/CT(2214个,26.76%)。在单核苷酸重复基元中,以A/T为主,占单核苷酸重复总数的96.64%。在二核苷酸重复基元中,以AG/CT为主,占二核苷酸重复总数的82.77%,其次分别为AT/AT和AC/GT,分别占二核苷酸重复总数的11.44%和5.61%。在三核苷酸重复基元中,以AAG/CTT为主,占三核苷酸重复总数的32.04%,其次为AGC/CTG、ATC/ATG、AGG/CCT、AAT/ATT和ACC/GGT,分别占三核苷酸重复总数的5.16%、4.93%、3.51%、3.48%和3.48%。在四核苷酸重复基元中,以AAAG/CTTT出现频率最高,占四核苷酸重复总数的20.43%,其次为AAAT/ATTT和AAAC/GTTT,分别占四核苷酸重复总数的18.28%和10.75%。各类五和六核苷酸重复出现频率均较低。
表 2 辣木转录组SSR基序类型分布Table 2. Distribution of SSR types in transcriptome of M. oleiferaSSR基序 数量 A/T 4452 C/G 155 AG/CT 2214 AT/AT 306 AC/GT 150 CG/CG 5 AAG/CTT 298 ATC/ATG 132 AGC/CTG 138 AGG/CCT 94 AAT/ATT 93 ACC/GGT 93 AAC/GTT 46 ACG/CGT 19 AAAAG/CTTTT 2 AAACC/GGTTT 1 AACAAG/CTTGTT 1 AAGCAG/CTGCTT 1 ACCATC/ATGGTG 1 ACCCTG/AGGGTC 1 ACTAGC/AGTGCT 1 ACT/AGT 3 CCG/CGG 14 AAAG/CTTT 13 AAAT/ATTT 17 AAGC/CTTG 1 AAGG/CCTT 2 AATT/AATT 1 ACAG/CTGT 1 ACAT/ATGT 3 AGCC/CTGG 1 AGCG/CGCT 1 AGGC/CCTG 1 AGGG/CCCT 3 ATCC/ATGG 1 AAAAC/GTTTT 1 AAATC/ATTTG 1 AACTGC/AGTTGC 1 ACCAGC/CTGGTG 1 ACCATG/ATGGTC 1 ACGATC/ATCGTG 1 AGCAGG/CCTGCT 1 3. 讨论与结论
本研究对来自辣木叶片的15 824条Unigene进行SSR位点分析,检测到SSR位点8 272个,出现频率为52.28%,略低于李54.51%[20],显著高于连翘(25.15%)[30]、刺梨(20.37%)[21]、青稞(19.58%)[22]、野三七(16.86%)[33]、文冠果(12.93%)[23]、蜡梅(12.35%)[34]、红掌(12.17%)[35]、布渣叶(12.14%)[24]、白姜(9.39%)[25]、辣椒(7.84%)[26]、洋葱(5.57%)[27]、红松(3.74%)[23]和冷蒿(2.51%)[28]。本研究中SSR检出频率高达52.28%,表明辣木叶片转录组中含有丰富的SSR,可为SSR标记的开发提供丰富的候选位点。值得注意的是,本研究仅选择序列长度大于1 kb的Unigene进行分析,这可能是导致SSR检测频率较高的原因之一。方智振等[20]采用这种策略在李转录组中搜索到大量的SSR。王兴春等[29]在连翘转录组分析中也采用了相同的策略,但SSR出现频率显著低于本研究。刺梨、青稞、白姜、红松和冷蒿等植物的研究中均对所有Unigene进行分析,但SSR出现频率存在显著差异[21, 23, 25, 28]。此外,SSR位点识别参数也是影响结果的重要因素。本研究对单核苷酸、二核苷酸、三核苷酸、四核苷酸、五核苷酸和六核苷酸重复SSR进行分析,而文冠果[23]、布渣叶[24]、白姜[25]、冷蒿[28]和野三七[33]等仅对二核苷酸至六核苷酸SSR进行分析。然而,布渣叶[24]、白姜[25]和野三七[33]设置的参数一致,但结果也存在显著的差异。因此,植物转录组中SSR的发生频率受物种、转录组数据量和检索标准等因素的影响[20]。
在辣木叶片转录组中,单核苷酸重复(53.82%)为主要SSR类型,其次为二核苷酸重复(32.34%)和三核苷酸重复(11.24%)。这与李[20]、苦荞[30]和海甘蓝[31]等植物的结果类似,但不同于红掌[35]、荔枝[41]和党参[42]等以二核苷酸重复为主的植物。与党参[32]、马铃薯[33]、蜡梅[34]和李[20]等植物相似,辣木转录组中二核苷酸重复以AG/CT重复为主,AT/AT和AC/GT次之,出现频率极低的为CG/CG。但该结果不同于以AC/TG重复为主的洋葱[27]和冷蒿[28]等植物。董清华等[34]认为双子叶植物中主要三核苷酸重复为AAG/CTT的观点,在本研究得到了验证。在单子叶植物玉米、水稻和大麦中的三核苷酸重复分别为CCG/GGC、AGG/TCC和CCG/GGC[34],与双子叶植物有明显的差异。可见,不同物种的主要SSR类型明显不同。
本研究分析了辣木转录组SSR的分布、结构特点、基序重复类型和频率特征,并与其他植物的研究结果进行了比较。结果表明辣木转录组中含有大量SSR,且类型多样,可为辣木SSR引物开发以及种质资源多样性、品种鉴定及亲缘关系研究等奠定良好的基础。
-
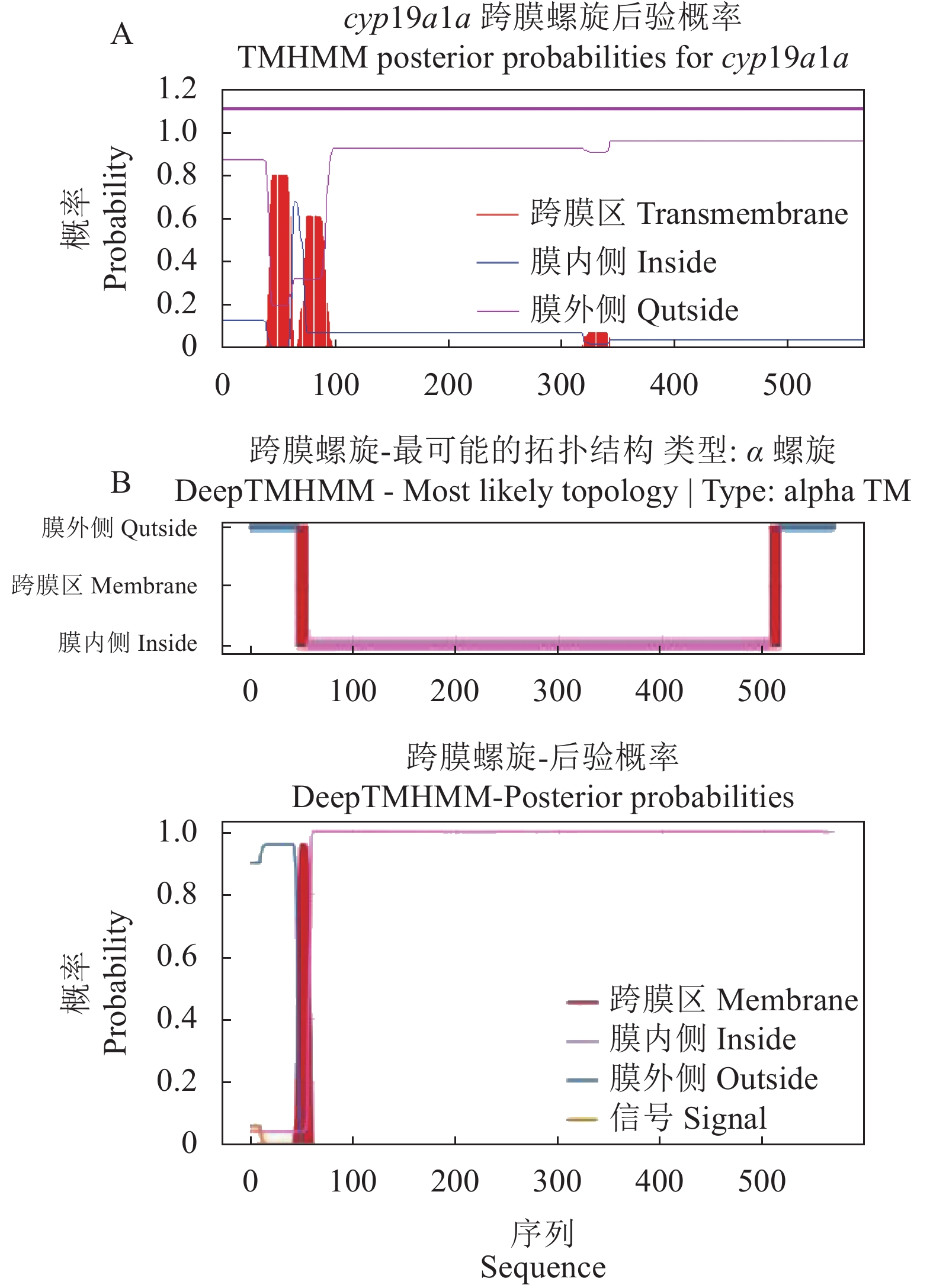
图 3 不同脊椎动物的Cyp19a1a氨基酸的同源性分析
阴影区Ⅰ~Ⅴ依次表示跨膜螺旋区、I-螺旋区、Ozol’s肽区、芳香化酶特异保守区、血红素结合区。“*”表示保守的氨基酸残基,“:”表示保守替换的氨基酸残基,“.”表示相似性氨基酸。
Figure 3. Homology of Cyp19a1a in different vertebrates
Shadow regions I–V represent transmembrane helix region, I-helix region, Ozol’s peptide region, aromatase specific conserved region, and heme-binding region, respectively. “*”indicates highly conserved amino acid residue; “:” indicates conservative amino acid residue replaced; “.” represents similar amino acids.
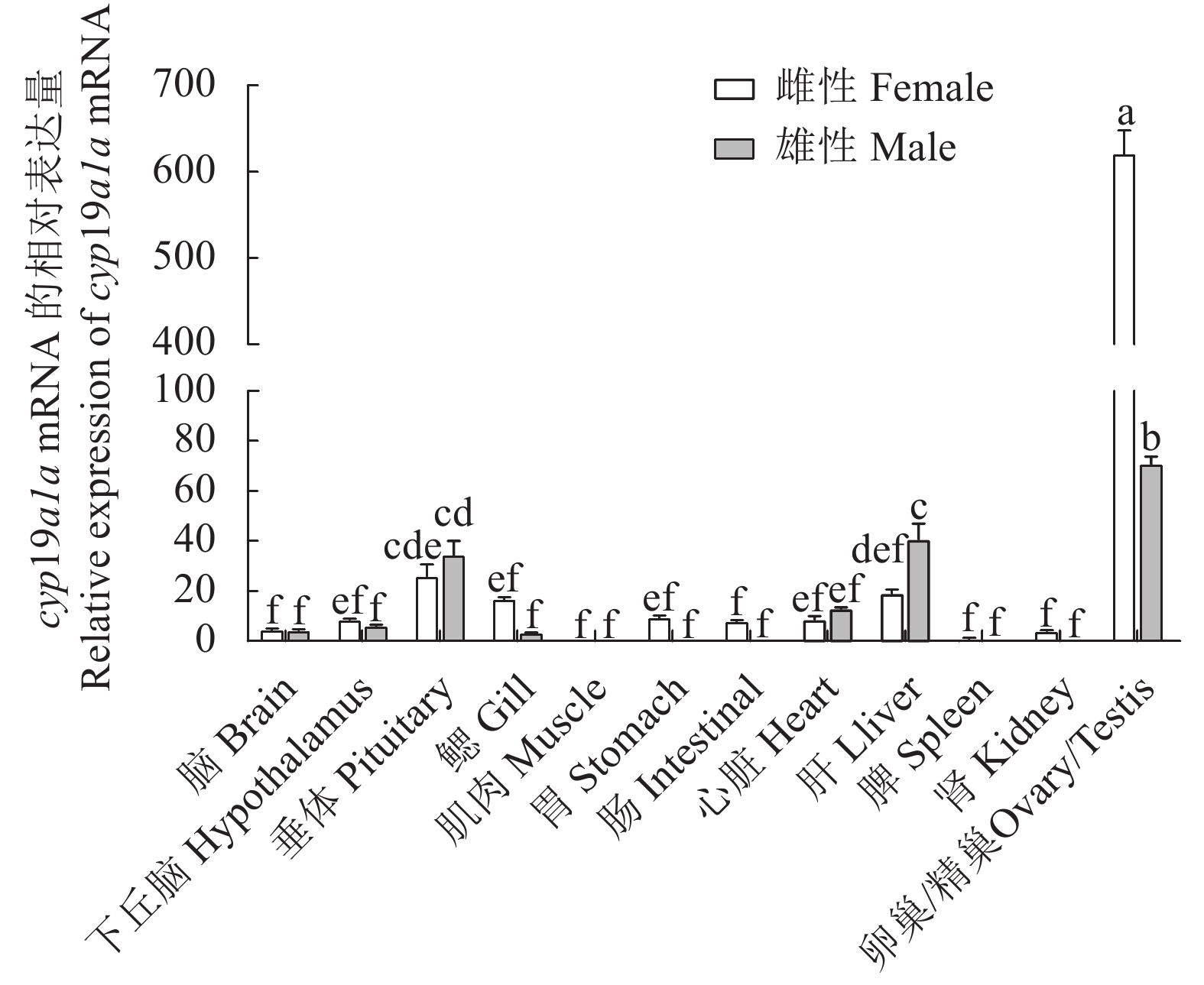
图 5 cyp19a1a基因在3龄雌雄兰州鲇不同组织表达水平
不同字母表示所有性别组织之间差异显著(P < 0.05),相同字母表示差异不显著(P > 0.05)。
Figure 5. cyp19a1a expressions in different organs of 3-year-old female and male S. lanzhouensis
Different letters mean significant difference at P<0.05 among all gender groups; identical letters mean no significant difference (P>0.05) .
图 6 兰州鲇cyp19a1a基因在不同年龄段性腺发育差异表达
1:3月龄;2:1龄;3:3龄;不同字母表示所有年龄段组织间差异显著(P < 0.05),相同字母表示差异不显著(P > 0.05)。
Figure 6. cyp19a1a expressions during gonad development of S. lanzhouensis at different ages
1: 3-month-old; 2: 1-year-old; 3: 3-year-old; different letters mean significant difference at P<0.05; same letters mean no significant difference P>0.05.
图 7 Cyp19a1a在兰州鲇卵巢和精巢的细胞定位
1~3:3月龄卵巢细胞定位;5~7:1龄卵巢细胞定位;9~11:3龄卵巢细胞定位;13~15:3月龄精巢细胞定位;17~19:1月龄精巢细胞定位;21~23:3龄精巢细胞定位;4、8、12、16、20、24:3月龄卵巢、1龄卵巢、3龄卵巢、3月龄精巢、1龄精巢、3龄精巢阴性对照;OG:卵原细胞;PO:初级卵母细胞;MC:间质细胞;黄色箭头:滤泡膜;蓝色箭头:放射膜;红色箭头:初级卵母细胞小生长期;绿色箭头:鞘膜细胞;棕色表示阳性信号;NC表示阴性对照;图1、5、9、13、17、21为4X;图2、6、8、10、14、18、20、22为20X;图3、4、7、11、12、15、16、19、23、24为40X。
Figure 7. Cellular localizations of Cyp19a1a in ovary and testis of S. lanzhouensis
1–3: cellular localization of 3-month-old ovary; 5–7: cellular localization of 1-year-old ovary; 9–11: cellular localization of 3-year-old ovary; 13–15: cellular localization of 3-month-old testis; 17–19: cellular localization of 1-year-old testis; 21–23: cellular localization of 3-year-old testis; 4, 8, 12, 16, 20, and 24: negative control of 3-month-old ovary, 1-year-old ovary, 3-year-old ovary, 3-month-old testis, 1-year-old testis, and 3-year-old testis, respectively; OG: oogonia; PO: primary oocyte; MC: interstitial cells; Yellow arrow: follicular membrane; Blue arrow: radioactive film; Red arrow: primary oocyte niche growth phase; Green arrow: sheath cells; Brown indicates positive signal; NC: negative control. Figs. 1, 5, 9, 13, 17, and 21 in scale of 4X; Figs. 2, 6, 8, 10, 14, 18, 20, and 22 in scale of 20X; Figs. 3, 4, 7, 11, 12, 15, 16, 19, 23, and 24 in scale of 40X.
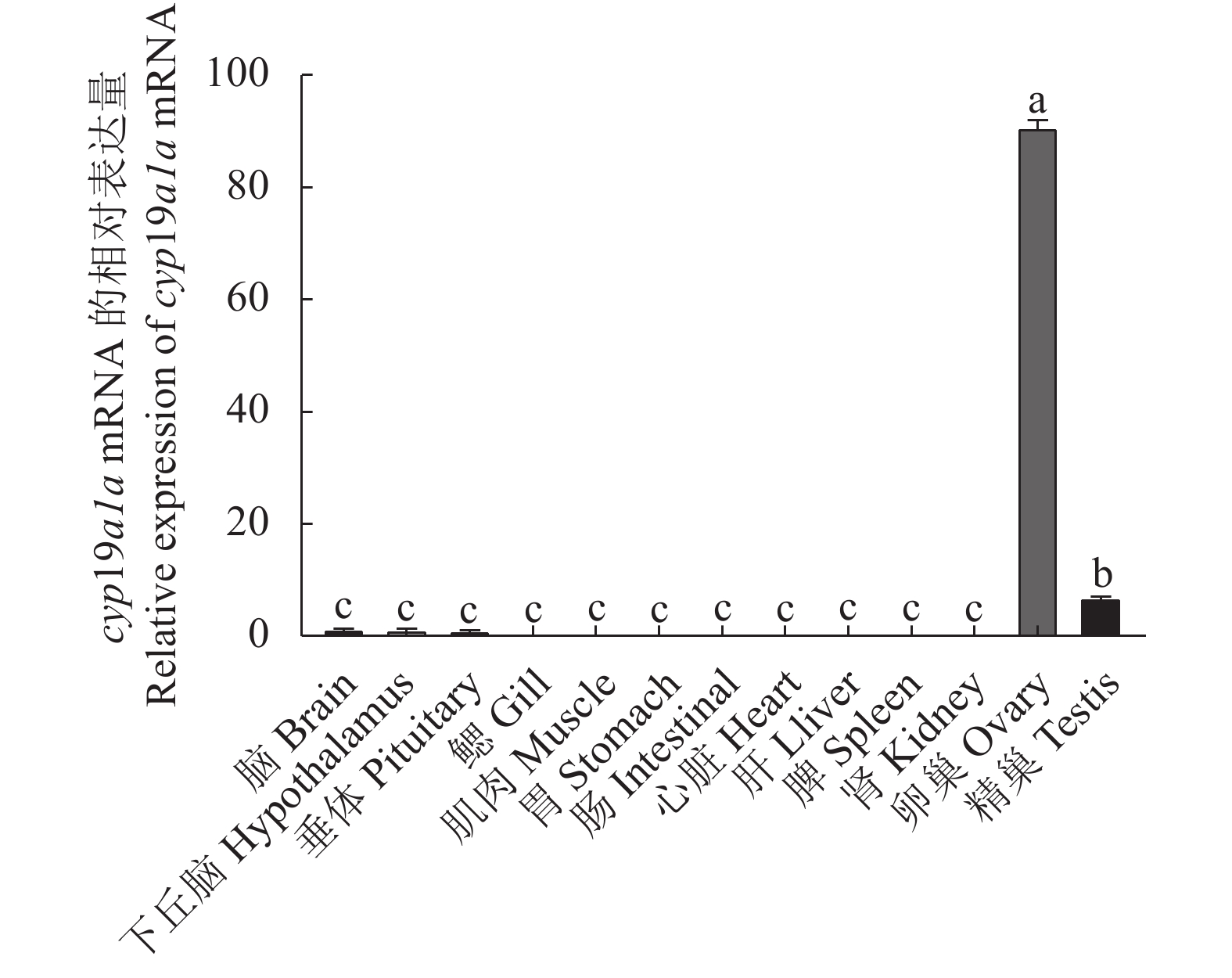
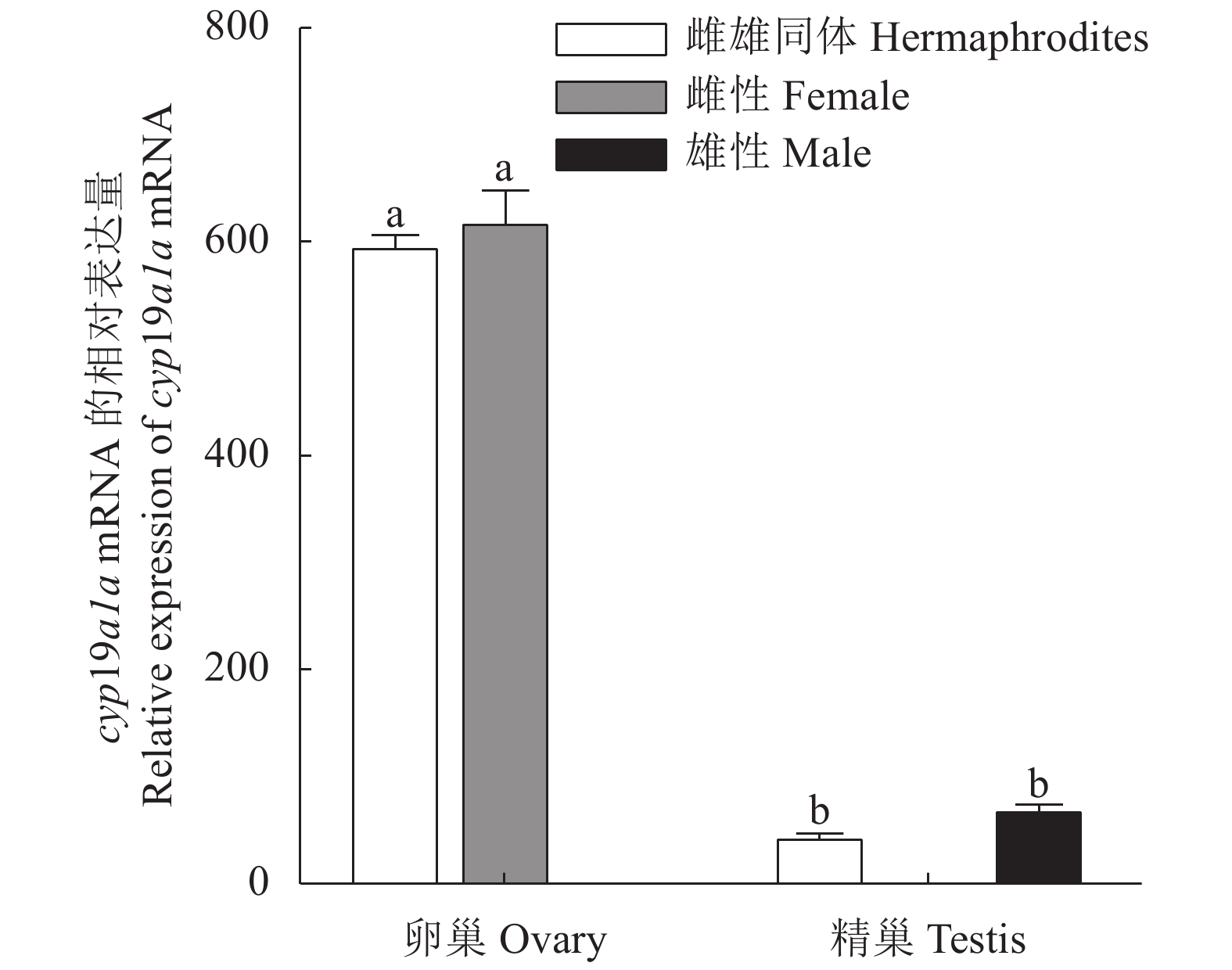
图 9 cyp19a1a基因在3龄兰州鲇雌雄同体和雌雄异体性腺表达水平
不同字母表示所有性别之间差异显著(P < 0.05),相同字母表示差异不显著(P > 0.05)。
Figure 9. Expression of cyp19a1a in gonads of 3-year-old hermaphroditic and gonochoristic S. lanzhouensis
Different letters mean significant difference at P<0.05 among gender groups; same letters mean no significant difference at P>0.05.
表 1 本研究所用引物
Table 1 Primers applied
引物名称
Primer name序列(5′-3′)
Sequence(5′-3′)用途
Usagecyp19a1a-F TACTGCTGCTGTGTCTGGTT 中间序列克隆 Intermediate sequence cloning cyp19a1a-R CGTTGGTGTGTGCGATGTT 中间序列克隆 Intermediate sequence cloning cyp19a1a-qF CTTCAGGACAAGCACAGGAAC 荧光定量PCR Fluorescence quantitative PCR cyp19a1a-qR GGAGCCGCAATCACCATCT 荧光定量PCR Fluorescence quantitative PCR β-actin F AAGATCATTGCCCCACCTGA 荧光定量PCR Fluorescence quantitative PCR β-actin R CCTGCTTGCTGATCCACATC 荧光定量PCR Fluorescence quantitative PCR cyp19a1a-5gsp1 ACCCGAACCGAAAGGCTGGAAAAAACGA 5′RACE-PCR cyp19a1a-5gsp2 CCACTGATCCACACTCGCACCACGTCCC 5′RACE-PCR cyp19a1a-3gsp1 TTTCCAGCCTTTCGGTTCGGGTCCTCGT 3′RACE-PCR cyp19a1a-3gsp2 GGCACTAACACACACCCCCACTGTCCTC 3′RACE-PCR UPM CTAATACGACTCACTATAGGGCAAGCAGTGGTATCAACGCAGAGT RACE通用引物 RACE universal primer UPM short primer CTAATACGACTCACTATAGGGC RACE通用引物 RACE universal primer -
[1] CAPEL B. Vertebrate sex determination: Evolutionary plasticity of a fundamental switch [J]. Nature Reviews Genetics, 2017, 18(11): 675−689. DOI: 10.1038/nrg.2017.60
[2] LI X Y, GUI J F. Diverse and variable sex determination mechanisms in vertebrates [J]. Science China Life Sciences, 2018, 61(12): 1503−1514. DOI: 10.1007/s11427-018-9415-7
[3] MEI J, GUI J F. Genetic basis and biotechnological manipulation of sexual dimorphism and sex determination in fish [J]. Science China Life Sciences, 2015, 58(2): 124−136. DOI: 10.1007/s11427-014-4797-9
[4] KOCOUR M, LINHART O, GELA D. Results of comparative growing test of all-female and bisexual population in two-year-old commoncarp (Cyprinus carpio L. ) [J]. Aquaculture International, 2003, 11(4): 369−378. DOI: 10.1023/A:1025728921552
[5] BYE V J, LINCOLN R F. Commercial methods for the control of sexual maturation in rainbow trout (Salmo gairdneri R. ) [J]. Aquaculture, 1986, 57(1/2/3/4): 299−309.
[6] FISCHER A J, THOMPSON B A. The age and growth of southern flounder, Paralichthys lethostigma, from Louisiana estuarine and offshore waters [J]. Bulletin of Marine Science, 2004, 75(1): 63−77.
[7] CHEN S L, LI J, DENG S P, et al. Isolation of female-specific AFLP markers and molecular identification of genetic sex in half-smooth tongue sole (Cynoglossus semilaevis) [J]. Marine Biotechnology (New York, N Y ), 2007, 9(2): 273−280. DOI: 10.1007/s10126-006-6081-x
[8] DAN C, MEI J, WANG D, et al. Genetic differentiation and efficient sex-specific marker development of a pair of Y- and X-linked markers in yellow catfish [J]. International Journal of Biological Sciences, 2013, 9(10): 1043−1049. DOI: 10.7150/ijbs.7203
[9] BEARDMORE J A, MAIR G C, LEWIS R I. Monosex male production in finfish as exemplified by tilapia: Applications, problems, and prospects [J]. Aquaculture, 2001, 197(1/2/3/4): 283−301.
[10] PAN Z J, LI X Y, ZHOU F J, et al. Identification of sex-specific markers reveals male heterogametic sex determination in Pseudobagrus ussuriensis [J]. Marine Biotechnology (New York, N Y ), 2015, 17(4): 441−451. DOI: 10.1007/s10126-015-9631-2
[11] SIMPSON E R, MAHENDROO M S, MEANS G D, et al. Aromatase cytochrome P450, the enzyme responsible for estrogen biosynthesis [J]. Endocrine Reviews, 1994, 15(3): 342−355.
[12] 李冰玉, 温海深, 王灵钰, 等. 花鲈性腺分化组织学及性别相关基因cyp11b和cyp19a1a的表达分析 [J]. 渔业科学进展, 2021, 42(6):185−193. LI B Y, WEN H S, WANG L Y, et al. Histology of gonadal differentiation and expression analysis of sex-related genes cyp11b, cyp19a1a in spotted sea bass(Lateolabrax maculatus) [J]. Progress in Fishery Sciences, 2021, 42(6): 185−193.(in Chinese)
[13] 陶敏, 刘少军, 张卓慧, 等. 不同倍性鲫鲤性腺型芳香化酶cyp19a1a基因cDNA的克隆及表达 [J]. 水产学报, 2014, 38(9):1201−1210. TAO M, LIU S J, ZHANG Z H, et al. Molecular cloning and comparative expression patterns of cyp19a1a of gene in different ploidy cyprinid fishes [J]. Journal of Fisheries of China, 2014, 38(9): 1201−1210.(in Chinese)
[14] 曹文怡, 易少奎, 杨楠, 等. 泥鳅cyp19a1基因克隆及其在倍性间的表达差异 [J]. 华中农业大学学报, 2021, 40(4):166−176. CAO W Y, YI S K, YANG N, et al. Molecular cloning and differential expression of cyp19a1 in tetraploid and diploid loach, Misgurnus anguillicaudatus [J]. Journal of Huazhong Agricultural University, 2021, 40(4): 166−176.(in Chinese)
[15] SAWYER S J, GERSTNER K A, CALLARD G V. Real-time PCR analysis of cytochrome P450 aromatase expression in zebrafish: Gene specific tissue distribution, sex differences, developmental programming, and estrogen regulation [J]. General and Comparative Endocrinology, 2006, 147(2): 108−117. DOI: 10.1016/j.ygcen.2005.12.010
[16] ZHANG Y, ZHANG W M, YANG H Y, et al. Two cytochrome P450 aromatase genes in the hermaphrodite ricefield eel Monopterus albus: MRNA expression during ovarian development and sex change [J]. The Journal of Endocrinology, 2008, 199(2): 317−331. DOI: 10.1677/JOE-08-0303
[17] ZHANG Y, ZHANG W M, ZHANG L H, et al. Two distinct cytochrome P450 aromatases in the orange-spotted grouper (Epinephelus coioides): CDNA cloning and differential mRNA expression [J]. The Journal of Steroid Biochemistry and Molecular Biology, 2004, 92(1/2): 39−50.
[18] FUKADA S, TANAKA M, MATSUYAMA M, et al. Isolation, characterization, and expression of cDNAs encoding the medaka (Oryzias latipes) ovarian follicle cytochrome P-450 aromatase [J]. Molecular Reproduction and Development, 1996, 45(3): 285−290. DOI: 10.1002/(SICI)1098-2795(199611)45:3<285::AID-MRD4>3.0.CO;2-O
[19] RUKSANA S, PANDIT N P, NAKAMURA M. Efficacy of exemestane, a new generation of aromatase inhibitor, on sex differentiation in a gonochoristic fish [J]. Comparative Biochemistry and Physiology Part C:Toxicology & Pharmacology, 2010, 152(1): 69−74.
[20] VIZZIANO D, BARON D, RANDUINEAU G, et al. Rainbow trout gonadal masculinization induced by inhibition of estrogen synthesis is more physiological than masculinization induced by androgen supplementation [J]. Biology of Reproduction, 2008, 78(5): 939−946. DOI: 10.1095/biolreprod.107.065961
[21] ZHANG X B, LI M R, MA H, et al. Mutation of foxl2 or cyp19a1a results in female to male sex reversal in XX Nile tilapia [J]. Endocrinology, 2017, 158(8): 2634−2647.
[22] 吴旭东, 张奇, 赵红雪, 等. 宁夏鲇属鱼类一新纪录种: 兰州鲇形态学特征描述 [J]. 淡水渔业, 2006, 36(3):26−29. DOI: 10.3969/j.issn.1000-6907.2006.03.005 WU X D, ZHANG Q, ZHAO H X, et al. A new species of catfish in ningxia—Silurus lanzhouensis and it's intensive morphological description [J]. Freshwater Fisheries, 2006, 36(3): 26−29.(in Chinese) DOI: 10.3969/j.issn.1000-6907.2006.03.005
[23] 邢露梅, 肖伟, 李兰兰, 等. 兰州鲇精液超低温冷冻保存技术研究及细胞损伤检测 [J]. 水生生物学报, 2021, 45(3):547−556. DOI: 10.7541/2021.2020.020 XING L M, XIAO W, LI L L, et al. Study of sperm cryopreservation in Silurus lanzhouensis and detection of cell damages after cryopreservation [J]. Acta Hydrobiologica Sinica, 2021, 45(3): 547−556.(in Chinese) DOI: 10.7541/2021.2020.020
[24] 连总强, 滚双宝, 李力, 等. 基于第二代测序技术兰州鲇线粒体基因组全序列测定与分析 [J]. 水生生物学报, 2017, 41(2):334−345. DOI: 10.7541/2017.41 LIAN Z Q, GUN S B, LI L, et al. Sequencing and analysis of the complete mitochondrial genome of Silurus lanzhouensis based on next generation sequencing technologies [J]. Acta Hydrobiologica Sinica, 2017, 41(2): 334−345.(in Chinese) DOI: 10.7541/2017.41
[25] 史丽娜, 张奇, 吴旭东, 等. 兰州鲇个体繁殖力的研究[J]. 甘肃农业大学学报, 2008, 43(1): 67-70. SHI L N, ZHANG Q, WU X D, et al. Individual fertility of the Silurus lanzhouensis[J]. Journal of Gansu Agricultural University, 2008, 43(1): 67-70. (in Chinese)
[26] 吴旭东, 连总强, 侯玉霞, 等. 兰州鲇野生群体和人工繁育群体遗传结构的比较研究 [J]. 淡水渔业, 2011, 41(3):34−38. DOI: 10.3969/j.issn.1000-6907.2011.03.006 WU X D, LIAN Z Q, HOU Y X, et al. Genetic structure analyses between wild population and artificial breeding ones of Silurus lanzhouensis [J]. Freshwater Fisheries, 2011, 41(3): 34−38.(in Chinese) DOI: 10.3969/j.issn.1000-6907.2011.03.006
[27] 魏大为, 连总强, 吴旭东, 等. 磁珠富集法筛选兰州鲇微卫星分子标记 [J]. 水生生物学报, 2014, 38(4):791−796. DOI: 10.7541/2014.110 WEI D W, LIAN Z Q, WU X D, et al. Microsatellite enrichment by magnetic beads in Silurus lanzhouensis [J]. Acta Hydrobiologica Sinica, 2014, 38(4): 791−796.(in Chinese) DOI: 10.7541/2014.110
[28] 连总强, 陈启发, 王燕, 等. 专用微胶囊饲料驯养培育兰州鲇稚幼鱼效果研究 [J]. 福建农业学报, 2020, 35(3):254−259. LIAN Z Q, CHEN Q F, WANG Y, et al. Microencapsulated forage for Silurus lanzhouensis larvae and juvenile aquaculture [J]. Fujian Journal of Agricultural Sciences, 2020, 35(3): 254−259.(in Chinese)
[29] 赛清云, 王远吉, 吴旭东, 等. 黄河鲇幼鱼对饲料蛋白和能量需要的初步研究 [J]. 淡水渔业, 2012, 42(4):53−58. DOI: 10.3969/j.issn.1000-6907.2012.04.010 SAI Q Y, WANG Y J, WU X D, et al. Preliminary studies on dietary protein and energy requirement of juvenile Silurus lanzhouensis [J]. Freshwater Fisheries, 2012, 42(4): 53−58.(in Chinese) DOI: 10.3969/j.issn.1000-6907.2012.04.010
[30] WANG T, LI Z, YU Z X, et al. Production of YY males through self-fertilization of an occasional hermaphrodite in Lanzhou catfish (Silurus lanzhouensis) [J]. Aquaculture, 2021, 539: 736622. DOI: 10.1016/j.aquaculture.2021.736622
[31] URBATZKA R, LUTZ I, KLOAS W. Aromatase, steroid-5-alpha-reductase type 1 and type 2 mRNA expression in gonads and in brain of Xenopus laevis during ontogeny [J]. General and Comparative Endocrinology, 2007, 153(1/2/3): 280−288.
[32] CAO M X, DUAN J D, CHENG N N, et al. Sexually dimorphic and ontogenetic expression of dmrt1, cyp19a1a and cyp19a1b in Gobiocypris rarus [J]. Comparative Biochemistry and Physiology Part A:Molecular & Integrative Physiology, 2012, 162(4): 303−309.
[33] DEVLIN R H, NAGAHAMA Y. Sex determination and sex differentiation in fish: An overview of genetic, physiological, and environmental influences [J]. Aquaculture, 2002, 208(3/4): 191−364.
[34] KITANO T, TAKAMUNE K, NAGAHAMA Y, et al. Aromatase inhibitor and 17alpha-methyltestosterone cause sex-reversal from genetical females to phenotypic males and suppression of P450 aromatase gene expression in Japanese flounder (Paralichthys olivaceus) [J]. Molecular Reproduction and Development, 2000, 56(1): 1−5. DOI: 10.1002/(SICI)1098-2795(200005)56:1<1::AID-MRD1>3.0.CO;2-3
[35] SMITH E K, GUZMÁN J M, LUCKENBACH J A. Molecular cloning, characterization, and sexually dimorphic expression of five major sex differentiation-related genes in a Scorpaeniform fish, sablefish (Anoplopoma fimbria) [J]. Comparative Biochemistry and Physiology Part B:Biochemistry and Molecular Biology, 2013, 165(2): 125−137. DOI: 10.1016/j.cbpb.2013.03.011
[36] 洪万树, 方永强. 鱼类芳香化酶活性研究的进展 [J]. 水产学报, 2000, 24(3):285−288. HONG W S, FANG Y Q. Advances on aromatase activity in fish [J]. Journal of Fisheries of China, 2000, 24(3): 285−288.(in Chinese)
[37] GUIGUEN Y, FOSTIER A, PIFERRER F, et al. Ovarian aromatase and estrogens: A pivotal role for gonadal sex differentiation and sex change in fish [J]. General and Comparative Endocrinology, 2010, 165(3): 352−366. DOI: 10.1016/j.ygcen.2009.03.002
[38] CALLARD G V. Autocrine and paracrine role of steroids during spermatogenesis: Studies in Squalus acanthias and Necturus maculosus [J]. Journal of Experimental Zoology, 1992, 261(2): 132−142. DOI: 10.1002/jez.1402610204
[39] DONG W, WILLETT K L. Local expression of CYP19A1 and CYP19A2 in developing and adult killifish (Fundulus heteroclitus) [J]. General and Comparative Endocrinology, 2008, 155(2): 307−317. DOI: 10.1016/j.ygcen.2007.05.018
[40] WANG X G, ORBAN L. Anti-Müllerian hormone and 11 beta-hydroxylase show reciprocal expression to that of aromatase in the transforming gonad of zebrafish males [J]. Developmental Dynamics:an Official Publication of the American Association of Anatomists, 2007, 236(5): 1329−1338. DOI: 10.1002/dvdy.21129
[41] NUNEZ B S, APPLEBAUM S L. Tissue- and sex-specific regulation of CYP19A1 expression in the Atlantic croaker (Micropogonias undulatus) [J]. General and Comparative Endocrinology, 2006, 149(2): 205−216. DOI: 10.1016/j.ygcen.2006.06.005
[42] TRANT J M, LEHRTER J, GREGORY T, et al. Expression of cytochrome P450 aromatase in the channel catfish, Ictalurus punctatus [J]. The Journal of Steroid Biochemistry and Molecular Biology, 1997, 61(3/4/5/6): 393−397.
[43] BELVEDERE P, DALLA VALLE L, LUCCHETTI A, et al. Extraglandular expression of genes encoding steroidogenic cytochromes in rainbow trout (Oncorhynchus mykiss walbaum)A [J]. Annals of the New York Academy of Sciences, 1998, 839(1): 589−591.
[44] IJIRI S, BERARD C, TRANT J M. Characterization of gonadal and extra-gonadal forms of the cDNA encoding the Atlantic stingray (Dasyatis sabina) cytochrome P450 aromatase (CYP19) [J]. Molecular and Cellular Endocrinology, 2000, 164(1/2): 169−181.
[45] KROON F J, MUNDAY P L, WESTCOTT D A, et al. Aromatase pathway mediates sex change in each direction [J]. Proceedings Biological Sciences, 2005, 272(1570): 1399−1405.
[46] DE MITCHESON Y S, LIU M. Functional hermaphroditism in teleosts [J]. Fish and Fisheries, 2008, 9(1): 1−43. DOI: 10.1111/j.1467-2979.2007.00266.x
[47] MANK J E, AVISE J C. Evolutionary diversity and turn-over of sex determination in teleost fishes [J]. Sexual Development, 2009, 3(2/3): 60−67.
[48] KUWAMURA T, KADOTA T, SUZUKI S. Bidirectional sex change in the magenta dottyback Pictichromis porphyrea: First evidence from the field in Pseudochromidae [J]. Environmental Biology of Fishes, 2015, 98(1): 201−207. DOI: 10.1007/s10641-014-0265-4
[49] MAXFIELD J M, COLE K S. Structural changes in the ovotestis of the bidirectional hermaphrodite, the blue-banded goby (Lythrypnus dalli), during transition from Ova production to sperm production [J]. Environmental Biology of Fishes, 2019, 102(11): 1393−1404. DOI: 10.1007/s10641-019-00914-2
[50] KOBAYASHI Y, KOBAYASHI T, NAKAMURA M, et al. Characterization of two types of cytochrome P450 aromatase in the serial-sex changing gobiid fish, Trimma okinawae [J]. Zoological Science, 2004, 21(4): 417−425. DOI: 10.2108/zsj.21.417
[51] ORLANDO E F, KATSU Y, MIYAGAWA S, et al. Cloning and differential expression of estrogen receptor and aromatase genes in the self-fertilizing hermaphrodite and male mangrove Rivulus, Kryptolebias marmoratus [J]. Journal of Molecular Endocrinology, 2006, 37(2): 353−365. DOI: 10.1677/jme.1.02101




 下载:
下载: