Effects of Degrading Hylocereus undatus Forests in Karst Rocky Desertification Area on Soil Ecoenzymatic Stoichiometry
-
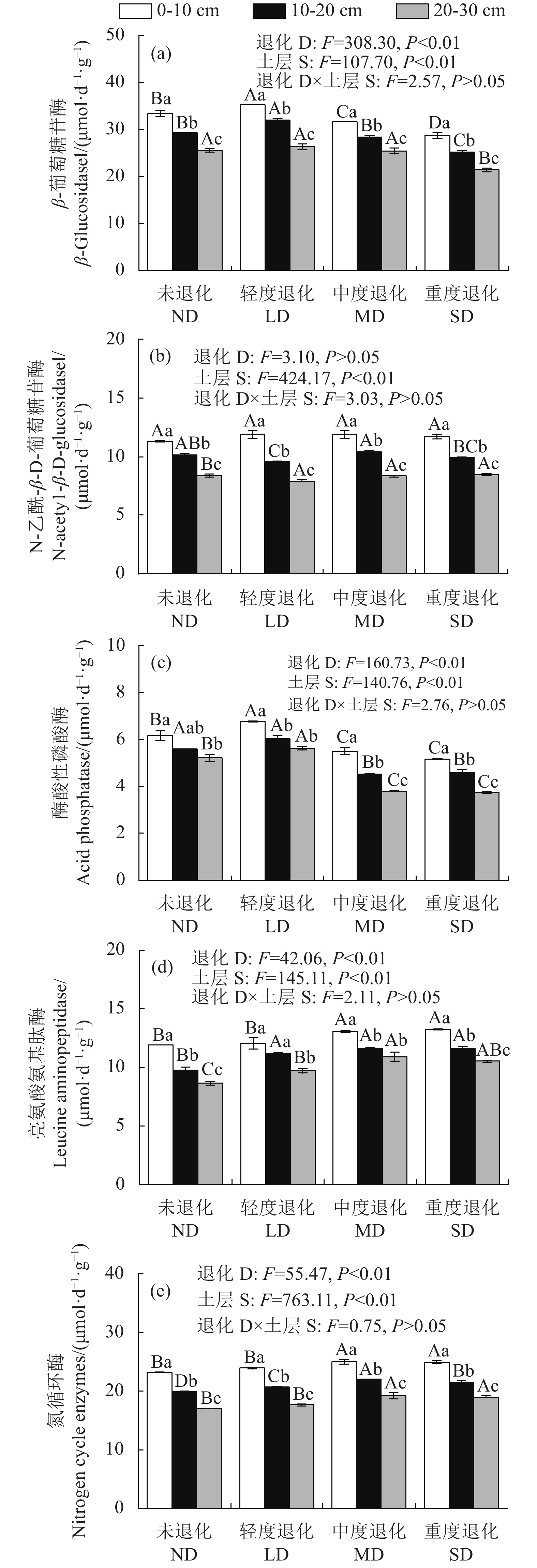
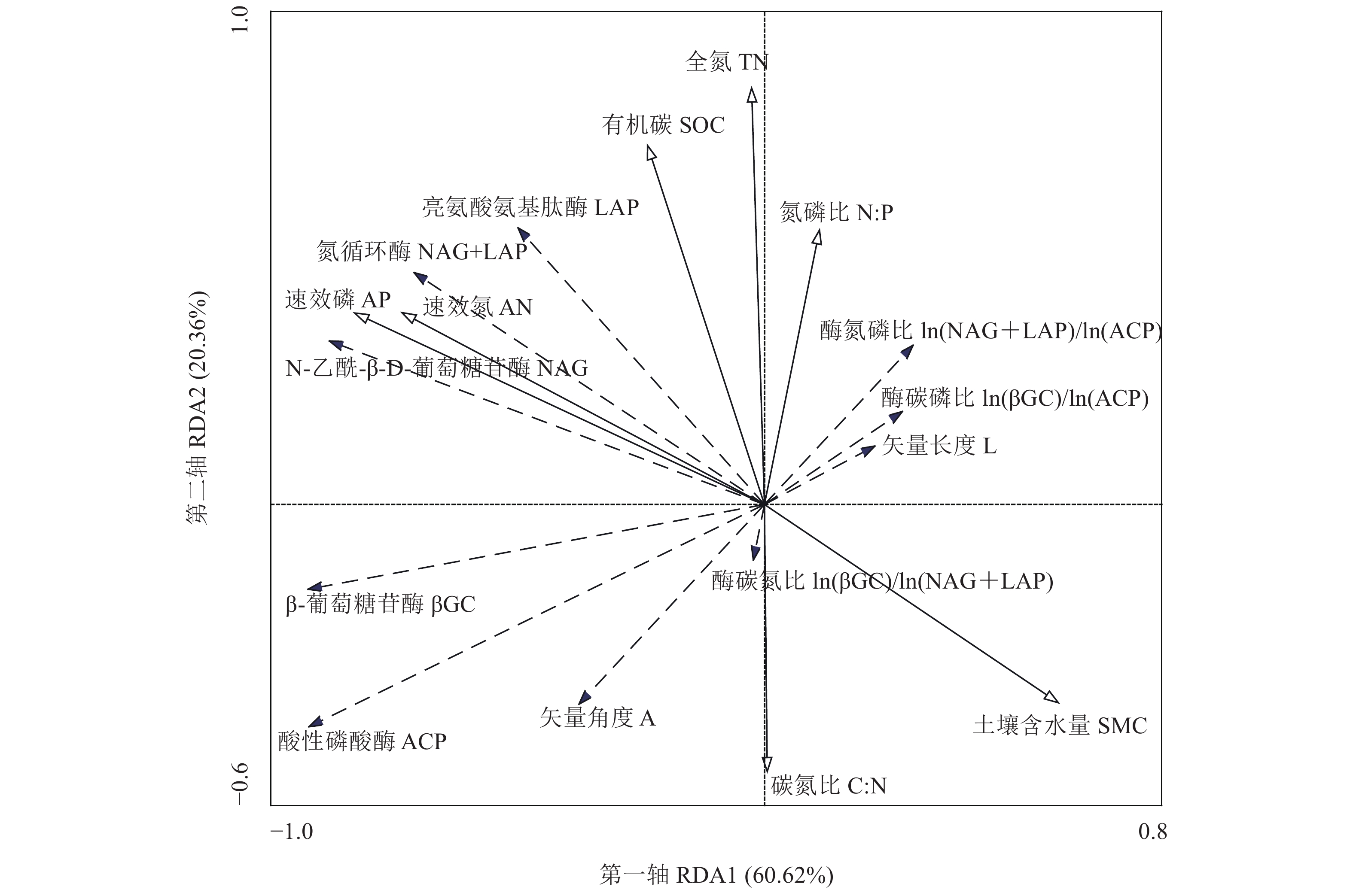
摘要:目的 探讨喀斯特石漠化地区火龙果林土壤酶活性和生态酶化学计量的变化特征。方法 以无退化(ND)、轻度退化(LD)、中度退化(MD)、重度退化(SD)4种不同退化火龙果(Hylocereus undatus)林为研究对象,采用单因素方差分析、双因素方差分析、皮尔逊相关分析和冗余分析(RDA)方法,研究0~30 cm 土层β-葡萄糖苷酶(βGC),N-乙酰-β-D-葡萄糖苷酶(NAG)、亮氨酸氨基肽酶(LAP)和酸性磷酸酶(ACP)4种土壤酶活性及生态酶化学计量特征的变异规律。结果 (1)土壤βGC和ACP总体上随退化加剧呈先升高后降低;NAG仅在10~20 cm和20~30 cm土层存在显著差异,而LAP表现为SD、MD显著大于ND;LAP+NAG活性总体呈上升趋势。(2)土壤酶C:N随退化加剧而降低,酶C:P和酶N:P均随退化加剧而一定程度增加,表明退化火龙果林资源利用策略发生一定改变。生态酶化学计量的矢量 L随退化加剧而变大,在 MD为最大值;矢量A随着退化加剧而降低,在SD为最小值,且矢量 A均小于45°,表明喀斯特石漠化地区火龙果林土壤微生物生长受N素限制。(3)RDA分析表明,土壤理化性质能够解释土壤酶及生态酶化学计量83.4%的变异,其中土壤AP和TN对土壤酶和生态酶化学计量影响最大,分别能够解释系统47.5%和24.3%的变异。结论 喀斯特石漠化地区火龙果林土壤微生物受N限制,且随退化加剧N限制有所增加。火龙果林退化对土壤酶活性和生态酶化学计量的影响是通过调控土壤N、P养分来实现的。Abstract:Objective Changes in soil enzyme activities and ecoenzymatic stoichiometry of the degrading Hylocereus undatus forests in karst rocky desertification areas were investigated.Method Pitaya growing areas with varying degrees of degradation, i.e., no degradation (ND), light degradation (LD), moderate degradation (MD), and severe degradation (SD), were targeted for the study. One-way ANOVA, two-way ANOVA, Pearson's correlation analysis, and redundancy analysis (RDA) were conducted to analyze the variations on the activities of β-glucosidase (βGC), N-acetyl-β-D-glucosidase (NAG), leucine aminopeptidase (LAP), and acid phosphatase (ACP) as well as the ecoenzymatic stoichiometry in 0–30 cm soil layers at the sampling areas.Result (1) Among the soil enzymes, βGC and ACP generally increased followed by a decline with increased degree of degradation. NAG differed significantly only in the soils in 10–20 cm and 20–30 cm layers. LAP was greater in SD and MD than ND area. In general, the LAP+NAG activities increased with the progress of degradation. (2) Soil enzyme C:N decreased with increasing degradation, but the ratios of C:P and N:P increased to some extent, indicating changes occurred on resource utilization by the degraded pitaya forest. As the degradation deepened, the vector L of ecoenzymatic stoichiometry enlarged to maximize in MD; the vector A decreased to bottom out in SD; and all vectors A remained less than 45°, which suggested that the microbial growth in soil was restricted by N availability. (3) An RDA analysis indicated that the soil physicochemical properties could explain 83.4% of the variation in soil enzyme and ecoenzymatic stoichiometry, and that AP and TN were the key factors that covered 47.5% and 24.3%, respectively, of the variation.Conclusion The microorganisms in soil of degraded H. undatus forests in karst rocky desertification areas were N-limited. Such nutrient restriction further aggravated the degradation. Conceivably, the enzyme activities and ecoenzymatic stoichiometry of the degraded pitaya forest soil could be remedied and rejuvenated by proper N and P fertilization.
-
0. 引言
【研究意义】香蕉(Musa acuminat L. AAA group cv. Brazilian)是热带地区的重要经济作物之一,被联合国粮农组织(FAO)定位为发展中国家仅次于水稻、小麦、玉米之后的第四大粮食作物。然而其根系浅生,易受旱,干旱胁迫极大地伤害香蕉正常的生理代谢活动,造成香蕉减产和品质下降,是香蕉产业中亟需解决的关键问题。【前人研究进展】香蕉水通道蛋白(Aquaporin,AQP)是生物体内广泛存在的一类位于各种细胞膜上24~34 kD的小分子跨膜蛋白[1-2],隶属于MIP(Major intrinsic protein, MIP)超家族,能够调节水分以及甘油、硼、二氧化碳等小分子物质的运输[3-4]。AQP在植物的生长发育中起着重要的作用[5-7],如种子萌发、细胞伸长、种子发芽、生殖生长和气孔运动等[8]。越来越多的研究表明,AQP能够维持植物中水的稳态响应各种非生物胁迫[4],主要包括冷害、盐害、旱害等。近年来的研究结果表明AQP能够提高植物的抗旱性,在植物中过表达OsPIP1、OsPIP2、VfPIP1、BnPIP1能够增加植物对渗透胁迫或干旱胁迫的耐受性[4]。过表达TaAQP7提高了转基因烟草的抗旱性[9]。用小麦TaTIP2; 2转化拟南芥提高了转基因植物的抗旱能力[10]。【本研究切入点】前期试验表明了MaAQP1提高了转基因拟南芥的抗旱性[11],而其提高植物抗旱性的作用机制仍未有报道。【拟解决的关键问题】本试验通过对干旱胁迫的香蕉进行cDNA合成及文库构建,克隆MaAQP1启动子并构建其诱饵载体,为进一步进行酵母单杂等研究MaAQP1响应干旱胁迫的作用机制奠定了基础。
1. 材料与方法
1.1 试验材料
1.1.1 植物材料
将5叶1心的巴西蕉(Musa acuminat L. AAA group cv. Brazilian)进行干旱胁迫处理[11],当土壤含水量为45%时取根、茎、叶一起混合冻样。
1.1.2 试验试剂
Plasmid Maxi Kit购自德国Qiagen公司,TRIzol Reagent购自美国Invitrogen公司,1 kb DNA Ladder和超纯琼脂糖购自上海海科生物技术有限公司,Taq DNA聚合酶等购自宝生物工程有限公司。cDNA文库构建试剂盒购自Clontech公司;Adenine Sulfate(Ade)为Amresco公司产品。各种工具酶、Marker购自TaKaRa公司。PCR引物合成和测序由华大完成。
1.2 试验方法
1.2.1 MaAQP1启动子克隆及序列分析
在香蕉全基因组测序[12]的基础上通过香蕉A基因组网址(http://banana-genome.cirad.fr/)获得MaAQP1的ATG起始密码子的2 kb的5′侧翼区域。通过引物从香蕉基因组DNA扩增序列,最后获得MaAQP1的翻译起始位点(ATG)的1 362 bp视为全长启动子,将其构建到pMD19-T载体上并测序确认。利用启动子顺式作用元件预测软件PlantCARE(http://bioinformatics.psb.ugent.be/webtools/plantcare/html/)对该启动子元件进行预测分析。
1.2.2 pHIS2-pMaAQP1诱饵载体构建
根据MaAQP1启动子序列分别在其两端添加Eco RI和Sac I的酶切位点序列设计引物,引物序列pMaAQP1-FAAGAATTCCTGCGACGGTTCGTAAAGAG;pMaAQP1-R:GGGAGCTCTGAGTGGAGGAATCAGGGTG,从pMD19T-pMaAQP1上进行扩增,将扩增条带回收,和pHIS2表达载体分别用Eco RI和Sac I进行双酶切,酶切产物分别纯化回收后进行连接,连接产物转化到E.coli DH5α感受态细胞中,活化后涂布于LB固体培养基上(内含30 ng·mL−1 Amp),37 ℃过夜培养后挑取单菌落摇菌,提取质粒进行酶切鉴定,然后选取阳性单克隆送华大基因测序,测序正确的即为诱饵载体pHIS2-pMaAQP1。
1.2.3 诱饵载体转化酵母菌株及自激活检测
将诱饵质粒pHIS2-pMaAQP1、阳性和阴性对照分别转化到酵母菌Y187感受态中,并涂布于平板SD-Trp-Leu-His + 50 mmol·L−1 3AT上生长。从SD-T平板中挑取单菌落于50 mL液体培养基中,在温度30 ℃、转速225 r·min−1培养18 h。将菌液转接于500 mL液体YPDA培养基中,使初始OD600 = 0.2,30 ℃,转速824 r·min−1 条件下培养4~5 h,至OD600=0.6。在转速1 737 r·min−1 下离心5 min集菌。依次用30 mL无菌H2O,转速1 000 r·min−1,室温下5 min集菌并弃上清。依次用20 mL 0.1 mol·L−1 LiAc,10 mL 0.1 mol·L−1 LiAc重复上一步骤,离心集菌弃上清。向离心管中依次加入1.44 mL 1 mol·L−1 LiAc,9.6 mL 50%PEG3350,25 μg文库质粒DNA,300 μL ssDNA(10 mg·mL−1)剧烈振荡1 min至完全混匀。在30 ℃水浴中孵育30 min。42 ℃水浴热激25 min。重复30 ℃水浴1 h。在室温下转速1 453 r·min−1 离心5 min离心集菌弃上清。每个转化用200 μL无菌H2O重悬菌体,温和混匀,并涂布于相应的缺陷型平板中,在30 ℃中培养3~4 d。
分别从表1中各转化的平板上各随机挑取3个单菌落,稀释后涂板至对应的不添加histidine、添加不同浓度(25、50 mmol·L−1)3AT的缺陷型平板上(3AT是酵母HIS3蛋白合成的竞争性抑制剂,用于抑制His3基因的泄漏表达),30 ℃恒温培养3 d。记录每块平板上的克隆数,并计算每个转化反应在缺陷平板上的转化子数量和生长率。
表 1 酵母转化反应Table 1. Yeast transformation reaction反应
ReactionAD质粒
AD plasmidBD质粒
BD plasmid转化平板
Conversion plate检测平板
Detection plate检测内容
Test content1 —— pHIS2-pMaAQP1 SD-T SD-TH+3AT 自激活检测 Self-activation detection 2 pGAD53m pHIS2 SD-TL SD-TLH+3AT 阴性对照 Negative control 3 pGAD53m pHIS2-p53 SD-TL SD-TLH+3AT 阳性对照 Positive control 1.2.4 用于构建文库的cDNA合成
用CTAB法提取干旱胁迫处理的香蕉组织,使用Oligotex mRNA Kits(Qiagen)分离纯化样本的mRNA。用Supscript double stand cDNA Kit分别进行cDNA第一链和第二链的合成,加5′接头(3个读码框,每个读码框连接一份,共3份),使用1%浓度的低熔点琼脂糖胶,电泳cDNA产物,切胶回收1 000 bp以上的片段,用14 μL的DEPC水溶解回收产物。1%琼脂糖凝胶电泳检测。
1.2.5 酵母单杂交文库构建
(1)cDNA与载体的连接 使用同源重组的方式,将7 μL cDNA与3 μL的经酶切处理线性化的pGADT7-Rec2(单杂交)载体,5 μL的Infusion重组酶和5 μL的H2O混匀。于50 ℃反应1 h,加入2 μL灭活的Proteinase K重组酶,78 μL无菌H2O至总体积为100 μL,加入1 μl Glycogen(20 μg·μL−1),375 μL 100% ethanol,50 μL 7.5 mol·L−1 NH4OAc,混匀并于−80 ℃ 1 h,在4 ℃下,转速6 948 r·min−1离心30 min。弃上清,加入150 μL 70%乙醇,在4 ℃下,转速6 948 r·min−1离心3 min,重复此步骤一次,去尽上清,在室温下将cDNA晾干5~1 min。用10 μL DEPC H2O重悬cDNA,瞬离2 s收集cDNA,置冰上。
(2)电转化大肠杆菌感受态细胞 每2.5 μL的重组产物转化50 μL感受态细胞,将1 mm电转杯(Bio-Rad)置于−80 ℃预冷30 min。将2.5 μL重组产物和50 μL感受态细胞加入电转杯,置冰上45 min。于电转化仪(Bio-Rad)上电击,电击后迅速向电转杯中加入LB培养基1 mL,然后取入到新的15 mL离心管,补足体积到5 mL,置于37 ℃,824 r·min−1~868 r·min−1离心培养至少1 h。培养结束后,将培养物依次稀释10、100、1 000、10 000倍,分别取10 μL稀释液涂平板,剩余培养物可4 ℃保存过夜,或者加入甘油至终浓度20%存于−80 ℃。
(3)构建文库质量鉴定 库容量的鉴定:取转化后菌原液10 μL稀释1 000倍后,从中取出10 μL涂布LB平板(含氨苄抗性),第2 d计数。库容量(CFU·mL−1)= 平板上的克隆数/10 ×1 000 ×103,文库总库容量(CFU)= 库容量×文库菌液总体积。插入片段大小鉴定:挑取平板上的单克隆,PCR扩增,电泳检测PCR产物大小。
2. 结果与分析
2.1 基因启动子的克隆
如图1所示,根据香蕉A基因组网站(http://banana-genome.cirad.fr)设计启动子的引物进行扩增,扩增长度为1 362 bp。
2.2 基因启动子的序列分析
通过plantcare和PLACE(www.dna.affrc.go.jp)分析其顺式作用元件,结果显示了1 362 bp的启动子序列中共有72个顺式作用元件,包括了TATA-box和CAAT-box核心元件,ABA响应元件ABRE、MYB元件、MYC元件、ERE元件、MeJA响应元件,BOX II、G-box、GT1-motif、I-BOX等光响应元件,以及分生组织响应元件等(表2)。
表 2 MaAQP1启动子元件分析Table 2. Element analysis on MaAQP1 promoter序号
Number结合位点
TFBs元件功能
Function of the motif器官
Organism序列
Sequence位置
Position1 AAGAA-motif LRE 燕麦
Avena sativaGAAAGAA/gGTAAAGAAA −1 172,−1 178 2 ABRE 脱落酸响应
Abscisic acid responsiveness拟南芥
Arabidopsis thalianaACGTG −55,−863 3 AT~TATA-box LRE 拟南芥
Arabidopsis thalianaTATATA −319,−343,−331,−355,−327,−353,−339,−363,
−323,−347,−335,−359,−329,−351,−341,−321,
−345,−333,−325,−337,−357,−349,−3614 Box II 光响应元件
light responsive element马铃薯
Solanum tuberosumTGGTAATAA −32 5 CAAT-box 核心启动子
Core promoter烟草/拟南芥/豌豆
Nicotiana glutinosa/Arabidopsis thaliana/Pisum sativumCAAT −43,−459,−315,−316,−125,−583,−1 197,
−208,−725,−421,−471,−1586 CAT-box 分生组织表达
meristem expression拟南芥
Arabidopsis thalianaGCCACT −254 7 CCAAT-box 结合位点
MYBHv1 binding site MYBHv1大麦
Hordeum vulgareCAACGG −935 8 CGTCA-motif 响应
MeJA-responsiveness MeJA大麦
Hordeum vulgareCGTCA −1 088 9 ERE 烯响应元件
ethylene-responsive element烟草
Nicotiana glutinosATTTTAAA −377,−775 11 GT1-motif 乙烯响应元件
light responsive element燕麦
Avena sativaGGTTAAT −65 12 I-box 光响应元件
light responsive element小麦/棉花
Triticum aestivum/
Gossypium hirsutumAAGATAAGGCT/AGATAAGG −611,−612 13 MYB SRE 拟南芥
Arabidopsis thalianaCAACCA −151 14 MYB recognition site SRE 拟南芥
Arabidopsis thalianaCCGTTG −878 15 MYC SRE 拟南芥
Arabidopsis thalianaCATTTG/CATGTG −111,−264 16 TATA-box 核心启动子元件
Core promoter element甘蓝/拟南芥
Brassica napus/ Arabidopsis thalianaATATAT/TATATA/taTATAAAtc/ATTATA −318,−326,−323,−320,−328,−324,−332,−319,
−327,−322,−321,−325,−330,−329,−333,−334,
−335,−336,−343,−344,−345,−346,−347,−348,
−349,−350,−351,−352,−353,−354,−355,
−356,−357,−358,−359,−363,−364,−365,
−389,−391,−837,−83817 TGACG-motif MeJA响应
MeJA-responsiveness大麦
Hordeum vulgareTGACG −58,−226 18 Unnamed__1 未知
unknown玉米
Zea maysCGTGG −864,−1 274 19 Unnamed__4 未知
unknown欧芹
Petroselinum hortenseCTCC −28,−1 016,−1 009,−1 353,−913,−1 255,
−1 013,−1 261,−92220 as-1 LRE 拟南芥
Arabidopsis thalianaTGACG −58,−226 2.3 诱饵载体的构建
取转化了诱饵载体的大肠杆菌菌液进行PCR扩增,将产物用1%琼脂糖凝胶进行电泳鉴定。并对扩增得到阳性条带的克隆质粒抽提和测序。测序结果正确,同时其菌落PCR结果如图2,条带正确,说明MaAQP1启动子片段连接到了表达载体pHIS2上。
2.4 诱饵载体转化酵母菌株及自激活检测
分别从3个不同转化反应的平板上各随机挑取3个单菌落,稀释后涂板至相应的不添加Histidine(组氨酸),添加不同浓度(25、50 mmol·L−1)的3AT(3-ami,2,4-triazole)缺陷型平板上,在30 ℃下培养3 d,结果如表3和图3所示。阳性对照随着3AT浓度增加,生长率会降低,与阴性对照有明显差别。阴性对照由于HIS3报告基因未被激活,在添加3AT的平板上生长明显减少,随着3AT浓度升高,转化子数量减少。自激活检测结果显示,在不添加3AT时,平板上的生长菌落3 424个,在分别添加了25 mmol·L−1和50 mmol·L−1 3AT的平板上,转化子的生长明显受到抑制,其生长比例与阴性对照的生长比例基本一致,表明其未激活HIS3报告基因。当添加25 mmol·L−1 3AT时,其生长受到抑制,菌落个数为408个,与对照的生长比例为11.9%,而当添加了50 mmol·L−1 3AT时,其菌落个数为68个,与对照的生长比例为1.9%。因此,后续试验可以用50 mmol·L−1 3AT进行。
表 3 自激活检测结果Table 3. Results of self-activation test反应
Reaction转化平板
Conversion plate检测平板
Detection plate检测内容
Test content3AT/(mmol·L−1) 0 25 50 生长数目
Growth number生长数目
Growth number25/0 生长比例
25/0 Growth ratio生长数目
Growth number50/0 生长比例
50/0 Growth ratio1 SD-T SD-TH 自激活检测
Self-activation detection3 424 408 11.9% 68 1.9% 2 SD-TL SD-TLH 阴性对照
Negative control3 968 1 376 34.6% 31 0.7% 3 SD-TL SD-TLH 阳性对照
Positive control5 024 5 136 102.2% 4 160 82.8% 2.5 cDNA文库的构建
将提取的干旱胁迫处理的香蕉总RNA,琼脂糖凝胶电泳检测,结果如图4中显示,总RNA的28S rRNA和18S rRNA条带清晰,条带28S亮度约为18S的2倍,说明总RNA质量较好,并无发生降解和污染。将RNA进行mRNA分离纯化,如图5所示,mRNA条带呈弥散状分布,分布均匀,mRNA总量为4.5 μg,可以满足建库需要。
使用同源重组的方法,将线性化的香蕉cDNA与经酶切处理线性化的pGADT7-Rec2(单杂交)载体混合共转化至大肠杆菌感受态中,取原液稀释后涂布于LB平板上(含氨苄抗性),第2 d计数,如图6所示,插入片段集中在500~2 000 bp,平均插入片段大于1 200 bp,阳性率为100%。在10 μL菌液稀释100倍后,取10 μL涂板,共生长了约250个克隆,其库容量为2.5×106 CFU·mL−1,共计5 mL的转化后原始菌液,则总库容量为1.25×107 CFU。
3. 讨论与结论
香蕉是大水大肥作物,干旱胁迫严重影响其生长发育及品质。目前的研究表明,水通道蛋白基因(AQP)作为膜蛋白能够增加植物的水分运输能力,提高植物的耐旱性。在拟南芥中,将AtPIP1; 2和AtPIP2; 2沉默会降低拟南芥抵御干旱的耐受性[13-14]。在水稻中过表达OsPIP1、OsPIP2和OsPIP1; 3能够增强植物对干旱胁迫的耐受性[15-16]。在烟草中过表达TaAQP7和TaAQP8可以提高植物对干旱的耐受性[3,9]。因此,研究AQP响应干旱胁迫的作用机制,对于进一步通过生物技术提高香蕉的耐旱能力具有重要的理论意义。本研究通过克隆干旱胁迫下的香蕉cDNA,构建用于酵母单杂交的cDNA文库,通过克隆香蕉水通道蛋白基因MaAQP1启动子,构建酵母诱饵表达载体,为下一步利用酵母单杂交筛选与香蕉MaAQP1互作的转录因子奠定了基础。
启动子在时间和空间意义上对于启动基因转录和调节基因表达都很重要。靶向基因及其启动子的遗传修饰可用于增强作物生长性能[17]。顺式元件与转录因子(TFs)之间的相互作用在转录的协调调控中对激活或抑制靶基因的表达起关键作用[18-19]。目前,可用于作物遗传改良的启动子很少。植物中最常见的启动子类型是35 s启动子CaMV35S,它可以在标记植物中驱动高水平的基因表达,而玉米泛素启动子则在单子叶植物中驱动基因表达。这些启动子能够在几乎所有组织和发育阶段的标志物或单子叶植物中驱动高水平的基因表达[20-22]。在特定条件下或某些组织中,诱导型或组织特异性启动子调节靶基因的表达。迄今为止,已经报道了一些植物的组织特异性和胁迫诱导型启动子。例如,拟南芥中的rd29A和rd29B启动子对高盐度和干旱有反应[23]。在小麦中,Dreb2启动子响应干旱胁迫[24]。来自芥菜植物的BjSOS2基因启动子在高盐,干旱和非生物胁迫以及其他形式的胁迫下起作用[25]。硬粒小麦基因TdPIP2; 1水稻及其启动子区域响应非生物胁迫[26]。本研究通过PlantCARE应用程序分析了MaAQP1启动子的顺式作用元件,元件包含了多个TATA-box和CAAT-box核心元件,以及与ABA和MeJA响应,MYB,MYC和光响应相关元件。TATA盒是在转录起始位点表达基因的核心启动子元件。CAAT-box是具有增强子活性的启动子中常见的顺式作用元件。但是,在MaAQP1启动子中未发现与干旱胁迫相关的反应元件。本研究发现ABRE是脱落酸(ABA)的顺式作用元件。研究表明ABA是一种重要的植物激素,参与许多重要的生物过程和反应,包括非生物胁迫。启动子的克隆及元件分析为下一步运用酵母单杂交鉴定直接调节MaAQP1启动子的转录因子,以及靶向转录因子调节MaAQP1表达的分子机制奠定了基础。
酵母单杂交cDNA文库的构建是酵母单杂交试验进行的基础,文库的质量好坏直接影响筛库过程。而cDNA文库的质量又是由转录成cDNA的mRNA决定的,因此必须对其质量进行严格的把关。本研究利用CTAB法提取香蕉组织RNA,RNA条带清晰无降解,进一步利用试剂盒分离纯化mRNA,合成香蕉cDNA,构建的cDNA文库,符合进一步试验的要求。另外,库容量以及插入片段的大小也直接影响到后期利用cDNA文库进行互作基因筛选的效率及结果,相关的研究表明,构建的木薯cDNA文库库容为1.5×106 CFU,插入片段大小为250~3 000 bp,能够为进一步的酵母单杂交筛选奠定良好的基础[27]。在本研究中,构建的文库库容为1.25×107 CFU,插入片段平均在1 200 bp左右,完全满足试验的要求。
-
图 1 不同退化火龙果林土壤理化性质
注:不同大写字母表示同一土层内不同退化程度间差异显著,不同小写字母表示同一退化程度不同土层间差异显著(P<0.05);F:重要性,D:退化,S:土层,D×S:退化与土层交互作用,图2~3同。
Figure 1. Physiochemical properties of pitaya forests with varied degrees of degradation
Note: Data with different capital letters indicate significant differences between varied degrees of degradation in same soil layer; data with different lowercase letters indicate significant differences between different layers of soil with same degree of degradation (p<0.05); F: Significance; D: degradation; S: soil layer, D×S: degradation and soil interaction; Same for Figs. 2 and 3.
表 1 火龙果林退化程度划分标准
Table 1 Classification standards of pitaya forest degradation
指标 Dividing index 无退化 ND 轻度退化 LD 中度退化 MD 重度退化 SD 平均株高 Average plant height/m >1.7 1.5~1.7 <1.5 <1.5 平均冠幅 Average crown width/m >2 1.8~2 <1.8 <1.5 枝条颜色 Branch color 绿色为主
Mainly green浅绿色为主
Mainly light green浅黄色为主
Mainly light yellow黄色为主
Mainly yellow平均结果枝条 Average result branches >40 <30 <20 <10 平均枝条长度 Average branch length/cm >130 110~130 <110 <90 平均枝条厚度 Average branch thickness/mm >6 <5 <5 <5 枯枝率 Rate of dead branches/% <10 >10 >20 >30 平均单果重 Average fruit weight/g >400 300~400 200~300 <200 -
[1] 刘艳, 宋同清, 蔡德所, 等. 喀斯特峰丛洼地不同土地利用方式土壤肥力特征 [J]. 应用生态学报, 2014, 25(6):1561−1568. LIU Y, SONG T Q, CAI D S, et al. Soil fertility characteristics under different land use patterns in depressions between karst hills [J]. Chinese Journal of Applied Ecology, 2014, 25(6): 1561−1568.(in Chinese)
[2] 喻阳华, 余杨, 杨苏茂, 等. 中国喀斯特高原山地区抗冻耐旱型植被退化现状及恢复对策 [J]. 世界林业研究, 2017, 30(1):72−75. YU Y H, YU Y, YANG S M, et al. Degradation situation and restoration countermeasures of frost resistant and drought tolerant vegetation in karst plateau mountain area, China [J]. World Forestry Research, 2017, 30(1): 72−75.(in Chinese)
[3] 高雨秋, 戴晓琴, 王建雷, 等. 亚热带人工林下植被根际土壤酶化学计量特征 [J]. 植物生态学报, 2019, 43(3):258−272. DOI: 10.17521/cjpe.2018.0299 GAO Y Q, DAI X Q, WANG J L, et al. Characteristics of soil enzymes stoichiometry in rhizosphere of understory vegetation in subtropical forest plantations [J]. Chinese Journal of Plant Ecology, 2019, 43(3): 258−272.(in Chinese) DOI: 10.17521/cjpe.2018.0299
[4] 彭子洋, 刘卫星, 田瑞, 等. 海拔和坡向对唐古拉山土壤胞外酶活性的影响 [J]. 生态学报, 2021, 41(19):7659−7668. PENG Z Y, LIU W X, TIAN R, et al. Effects of alitudle and aspect on soil extraellular enzyme activities in Tanggula Mountain [J]. Acta Ecologica Sinica, 2021, 41(19): 7659−7668.(in Chinese)
[5] HILL B H, ELONEN C M, JICHA T M, et al. Sediment microbial enzyme activity as an indicator of nutrient limitation in the great rivers of the Upper Mississippi River basin [J]. Biogeochemistry, 2010, 97(2): 195−209.
[6] 张星星, 杨柳明, 陈忠, 等. 中亚热带不同母质和森林类型土壤生态酶化学计量特征 [J]. 生态学报, 2018, 38(16):5828−5836. ZHANG X X, YANG L M, CHEN Z, et al. Patterns of ecoenzymatic stoichiometry on types of forest soils form different parent materials in subtropical areas [J]. Acta Ecologica Sinica, 2018, 38(16): 5828−5836.(in Chinese)
[7] 周玮, 周运超. 北盘江喀斯特峡谷区不同植被类型的土壤酶活性 [J]. 林业科学, 2010, 46(1):136−141. ZHOU W, ZHOU Y C. Soil enzyme activities under different vegetation types in Beipan River karst gorge district [J]. Scientia Silvae Sinicae, 2010, 46(1): 136−141.(in Chinese)
[8] 张建锋, 邢尚军. 环境胁迫下刺槐人工林地土壤退化特征研究 [J]. 土壤通报, 2009, 40(5):1086−1091. ZHANG J F, XING S J. Research on Soil degradation of Robinia pseudoacacia plantation under environmental stress [J]. Chinese Journal of Soil Science, 2009, 40(5): 1086−1091.(in Chinese)
[9] 蔡晓布, 张永青, 邵伟. 不同退化程度高寒草原土壤肥力变化特征 [J]. 生态学报, 2008, 28(3):1034−1044. CAI X B, ZHANG Y Q, SHAO W. Characteristics of soil fertility in alpine steppes at different degradation grades [J]. Acta Ecologica Sinica, 2008, 28(3): 1034−1044.(in Chinese)
[10] 于德良, 雷泽勇, 赵国军, 等. 土壤酶活性对沙地樟子松人工林衰退的响应 [J]. 环境化学, 2019, 38(1):97−105. YU D L, LEI Z Y, ZHAO G J, et al. Response of soil enzyme activity to the decline of Pinus sylvestris var. mongolica plantations on sand land [J]. Environmental Chemistry, 2019, 38(1): 97−105.(in Chinese)
[11] 李海云, 张建贵, 姚拓, 等. 退化高寒草地土壤养分、酶活性及生态化学计量特征 [J]. 水土保持学报, 2018, 32(5):287−295. LI H Y, ZHANG J G, YAO T, et al. Soil nutrients, enzyme activities and ecological stoichiometric characteristics in degraded alpine grasslands [J]. Journal of Soil and Water Conservation, 2018, 32(5): 287−295.(in Chinese)
[12] 喻岚晖, 王杰, 廖李容, 等. 青藏高原退化草甸土壤微生物量、酶化学计量学特征及其影响因素 [J]. 草地学报, 2020, 28(6):1702−1710. YU L H, WANG J, LIAO L R, et al. Soil microbial biomass, enzyme activities and ecological stoichiometric characteristics and influencing factors along degraded meadows on the Qinghai-Tibet Plateau [J]. Acta Agrestia Sinica, 2020, 28(6): 1702−1710.(in Chinese)
[13] GOENAGA R, MARRERO A, PÉREZ D. Yield and fruit quality traits of dragon fruit cultivars grown in puerto rico [J]. Horttechnology, 2020, 30(6): 803−808. DOI: 10.21273/HORTTECH04699-20
[14] 蒋忠诚, 李先琨, 曾馥平, 等. 岩溶峰丛山地脆弱生态系统重建技术研究 [J]. 地球学报, 2009, 30(2):155−166. JIANG Z C, LI X K, ZENG F P, et al. Study of fragile ecosystem reconstruction technology in the karst peak-cluster mountain [J]. Acta Geoscientica Sinica, 2009, 30(2): 155−166.(in Chinese)
[15] 熊康宁, 陈永毕, 陈浒, 等. 点石成金—喀斯特高原石漠化综合防治模式与技术[M]. 贵阳: 贵州科技出版社, 2011: 57-59. XIONG K N, CHEN Y B, CHEN H, et al. Midas touch: Technology and model of rocky desertification control in Guizhou [M]. Guiyang: Guizhou Science and Technology Press, 2011: 57-59. (in Chinese)
[16] YU Q H, WEI Y Y, LING M, et al. Physiological effect on Hylocereus undulatus and Hylocereus undatus under simulated karst soil water deficiency [J]. Journal of Resources and Ecology, 2015, 6(4): 269−275. DOI: 10.5814/j.issn.1674-764X.2015.04.011
[17] 罗志文, 李向宏, 彭超, 等. 红肉火龙果新品种‘大龙’的选育 [J]. 果树学报, 2018, 35(5):642−645. LUO Z W, LI X H, PENG C, et al. A new red pulp dragon fruit cultivar‘Dalong’ [J]. Journal of Fruit Science, 2018, 35(5): 642−645.(in Chinese)
[18] HO P L, TRAN D T, HERTOG M, et al. Effect of controlled atmosphere storage on the quality attributes and volatile organic compounds profile of dragon fruit (Hylocereus undatus) [J]. Postharvest Biology and Technology, 2021, 173: 111406. DOI: 10.1016/j.postharvbio.2020.111406
[19] 谢鸿根, 林旗华, 陈源, 等. 盐碱环境火龙果花、茎和果实氨基酸分析 [J]. 福建农业学报, 2017, 32(5):568−571. XIE H G, LIN Q H, CHEN Y, et al. Amino acids in flowers, stems and fruits of pitaya grown on saline habitats [J]. Fujian Journal of Agricultural Sciences, 2017, 32(5): 568−571.(in Chinese)
[20] HERNÁNDEZ-RAMOS L, GARCÍA-MATEOS M D, CASTILLO-GONZÁLEZ A M, et al. Fruits of the pitahaya Hylocereus undatus and H. ocamponis: nutritional components and antioxidants [J]. Journal of Applied Botany and Food Quality, 2020, 93: 197−203.
[21] 喻阳华, 钟欣平, 李红. 黔中石漠化区不同海拔顶坛花椒人工林生态化学计量特征 [J]. 生态学报, 2019, 39(15):5536−5545. YU Y H, ZHONG X P, LI H, et al. Ecological stoichiometry of Zanthoxylum planispinum var. dintanensis plantation at different altitudes in rocky desertification area of central Guizhou [J]. Acta Ecologica Sinica, 2019, 39(15): 5536−5545.(in Chinese)
[22] 王璐, 喻阳华, 秦仕忆, 等. 不同衰老程度顶坛花椒土壤养分质量的评价 [J]. 西南农业学报, 2019, 32(1):139−147. WANG L, YU Y H, QIN S Y, et al. Soil nutrient quality of Zanthoxylum planispinum trees with different aging level [J]. Southwest China Journal of Agricultural Sciences, 2019, 32(1): 139−147.(in Chinese)
[23] 王亚东, 魏江生, 周梅, 等. 大兴安岭南段不同生长衰退程度山杨林生态化学计量特征 [J]. 土壤通报, 2021, 52(4):854−864. WANG Y D, WEI J S, ZHOU M, et al. Ecological of stoichiometric characteristics of Populus davidiana forests with different growth and decline degrees in Southern Daxing'anling [J]. Chinese Journal of Soil Science, 2021, 52(4): 854−864.(in Chinese)
[24] 鲍士旦. 土壤农化分析[M]. 北京: 中国农业出版社, 2000: 20 − 114. [25] 焦鹏宇, 郭文, 陈泽龙, 等. 中亚热带不同林龄马尾松林土壤酶学计量特征[J/OL]. (2021-08-06). [2021-08-30]. https://doi. org/10.13227/j. hjkx. 202107043. JIAO P Y, GUO W, CHEN Z L, et al. Soil enzyme stoichiometric characteristics of Pinus massoniana plantations at different stand ages in mid-subtropical areas[J/OL]. (2021-08-06) [2021-08-30]. https://doi.org/10.13227/j.hjkx.202107043. (in Chinese)
[26] HILL B H, ELONEN C M, JICHA T M, et al. Ecoenzymatic stoichiometry and microbial processing of organic matter in northern bogs and fens reveals a common P-limitation between peatland types [J]. Biogeochemistry, 2014, 120(1): 203−224.
[27] 高阿娟, 刘子琦, 李渊, 等. 喀斯特峡谷区不同经济林地土壤水分变化特征—以贵州花江示范区为例 [J]. 中国岩溶, 2020, 39(6):863−872. GAO A J, LIU Z Q, LI Y, et al. Study on soil moisture variation characteristics of different economicforest lands in karst gorge area: A case study of Huajiang demonstration area in Guizhou Province [J]. Carsologica Sinica, 2020, 39(6): 863−872.(in Chinese)
[28] 徐国荣, 马维伟, 宋良翠, 等. 植被不同退化状态下尕海湿地土壤氮含量及酶活性特征 [J]. 生态学报, 2020, 40(24):8917−8927. XU G R, MA W W, SONG L C, et al. Characteristics of soil nitrogen content and enzyme activity in Gahai wetlandunder different vegetation degradation conditions [J]. Acta Ecologica Sinica, 2020, 40(24): 8917−8927.(in Chinese)
[29] 马维伟, 孙文颖. 尕海湿地植被退化过程中有机碳及相关土壤酶活性变化特征 [J]. 自然资源学报, 2020, 35(5):1250−1260. DOI: 10.31497/zrzyxb.20200519 MA W W, SUN W Y. Changes of organic carbon and related soil enzyme activities during vegetation degradation in Gahai Wetland [J]. Journal of Natural Resources, 2020, 35(5): 1250−1260.(in Chinese) DOI: 10.31497/zrzyxb.20200519
[30] 魏强, 王芳, 陈文业, 等. 黄河上游玛曲不同退化程度高寒草地土壤物理特性研究 [J]. 水土保持通报, 2010, 30(5):16−21. WEI Q, WANG F, CHEN W Y, et al. Soil physical characteristics on different degraded alpine grasslands in Maqu County in Upper Yellow River [J]. Bulletin of Soil and Water Conservation, 2010, 30(5): 16−21.(in Chinese)
[31] 李霞, 张小平, 喻晓, 等. 代森锰锌类农药对生姜种植地土壤酶活性及微生物群落结构的影响 [J]. 生态环境学报, 2016, 25(9):1569−1574. LI X, ZHANG X P, YU X, et al. Effect of mancozeb on soil enzyme activities and microbial community in ginger fields [J]. Ecology and Environmental Sciences, 2016, 25(9): 1569−1574.(in Chinese)
[32] PARIDA A K, DAS A B. Salt tolerance and salinity effects on plants: A review [J]. Ecotoxicology and Environmental Safety, 2005, 60(3): 324−349. DOI: 10.1016/j.ecoenv.2004.06.010
[33] 冯棣, 张俊鹏, 孙池涛, 等. 长期咸水灌溉对土壤理化性质和土壤酶活性的影响 [J]. 水土保持学报, 2014, 28(3):171−176. FENG D, ZHANG J P, SUN C T, et al. Effects of long-term irrigation with saline water on soil physical-chemical properties and activities of soil enzyme [J]. Journal of Soil and Water Conservation, 2014, 28(3): 171−176.(in Chinese)
[34] 李邵宇, 孙建, 王毅, 等. 青藏高原不同退化梯度草地土壤酶活性特征 [J]. 草业科学, 2020, 37(12):2389−2402. LI S Y, SUN J, WANG Y, et al. Characteristics of soil enzyme activities in different degraded gradient grasslands on the Tibetan Plateau [J]. Pratacultural Science, 2020, 37(12): 2389−2402.(in Chinese)
[35] QASWAR M, JING H, AHMED W, et al. Linkages between ecoenzymatic stoichiometry and microbial community structure under long-term fertilization in paddy soil: A case study in China [J]. Applied Soil Ecology, 2021, 161: 103860. DOI: 10.1016/j.apsoil.2020.103860
[36] 高小峰, 闫本帅, 吴春晓, 等. 长期施肥对黄土丘陵坡地农田土壤质量和谷子产量的影响 [J]. 干旱地区农业研究, 2021, 39(5):76−83. GAO X F, YAN B S, WU C X, et al. Effects of long-term fertilization on soil quality and millet yield on slope farmland in loess hilly areas [J]. Agricultural Research in the Arid Areas, 2021, 39(5): 76−83.(in Chinese)
[37] NGUYEN D B, ROSE M T, ROSE T J, et al. Effect of glyphosate and a commercial formulation on soil functionality assessed by substrate induced respiration and enzyme activity [J]. European Journal of Soil Biology, 2018, 85: 64−72. DOI: 10.1016/j.ejsobi.2018.01.004
[38] 喻岚晖. 藏北退化高寒草甸土壤酶化学计量学及微生物群落特征[D]. 杨凌: 中国科学院教育部水土保持与生态环境研究中心, 2021. YU L H. Soil enzyme stoichiometry and microbial community of degraded meadow on Northern Tibet[D]. Yangling: Research Center of Soil and Water Conservation and Eological Environment, Chinese Academy of Sciences and Mimistry Education, 2021. (in Chinese)
[39] 史丽娟, 王辉民, 付晓莉, 等. 中亚热带典型人工林土壤酶活性及其化学计量特征 [J]. 应用生态学报, 2020, 31(6):1980−1988. SHI L J, WANG H M, FU X L, et al. Soil enzyme activities and their stoichiometry of typical plantations in mid-subtropical China [J]. Chinese Journal of Applied Ecology, 2020, 31(6): 1980−1988.(in Chinese)
[40] VAN BRUGGEN A H C, SEMENOV A M. In search of biological indicators for soil health and disease suppression [J]. Applied Soil Ecology, 2000, 15(1): 13−24. DOI: 10.1016/S0929-1393(00)00068-8
[41] SINSABAUGH R L, HILL B H, FOLLSTAD SHAH J J. Ecoenzymatic stoichiometry of microbial organic nutrient acquisition in soil and sediment [J]. Nature, 2009, 462(7274): 795−798. DOI: 10.1038/nature08632
[42] SINSABAUGH R L, FOLLSTAD SHAH J J. Ecoenzymatic stoichiometry and ecological theory [J]. Annual Review of Ecology, Evolution, and Systematics, 2012, 43(1): 313−343. DOI: 10.1146/annurev-ecolsys-071112-124414
[43] GUO Z, ZHANG X, GREEN S M, et al. Soil enzyme activity and stoichiometry along a gradient of vegetation restoration at the Karst Critical Zone observatory in Southwest China [J]. Land Degradation & Development, 2019, 30(16): 1916−1927.
[44] WARING B G, WEINTRAUB S R, SINSABAUGH R L. Ecoenzymatic stoichiometry of microbial nutrient acquisition in tropical soils [J]. Biogeochemistry, 2014, 117(1): 101−113. DOI: 10.1007/s10533-013-9849-x
[45] XU Z W, YU G R, ZHANG X Y, et al. Soil enzyme activity and stoichiometry in forest ecosystems along the North-South Transect in eastern China (NSTEC) [J]. Soil Biology and Biochemistry, 2017, 104: 152−163. DOI: 10.1016/j.soilbio.2016.10.020
[46] 罗攀, 陈浩, 肖孔操, 等. 地形、树种和土壤属性对喀斯特山区土壤胞外酶活性的影响 [J]. 环境科学, 2017, 38(6):2577−2585. LUO P, CHEN H, XIAO K C, et al. Effects of topography, tree Species and soil properties on soil enzyme activity in karst regions [J]. Environmental Science, 2017, 38(6): 2577−2585.(in Chinese)
[47] 胡琛, 贺云龙, 黄金莲, 等. 神农架 4 种典型针叶人工林土壤酶活性及其生态化学计量特征 [J]. 林业科学研究, 2020, 33(4):143−150. HU C, HE Y L, HUANG J L, et al. Soil enzyme activity and its ecological stoichiometry in four typical coniferous planted forests in Shennongjia National Nature Reserve, China [J]. Forest Research, 2020, 33(4): 143−150.(in Chinese)
[48] 乔航, 莫小勤, 罗艳华, 等. 不同林龄油茶人工林土壤酶化学计量及其影响因素 [J]. 生态学报, 2019, 39(6):1887−1896. QIAO H, MO X Q, LUO Y H, et al. Patterns of soil ecoenzymatic stoichiometry and its influencing factors during stand development in Camellia oleifera plantations [J]. Acta Ecologica Sinica, 2019, 39(6): 1887−1896.(in Chinese)
[49] 孙彩丽, 王艺伟, 王从军, 等. 喀斯特山区土地利用方式转变对土壤酶活性及其化学计量特征的影响 [J]. 生态学报, 2021, 41(10):4140−4149. SUN C L, WANG Y W, WANG C J, et al. Effects of land use conversion on soil extracellular enzyme activity and its stoichiometric characteristics in karst mountainous areas [J]. Acta Ecologica Sinica, 2021, 41(10): 4140−4149.(in Chinese)
[50] ZHANG W, XU Y D, GAO D X, et al. Ecoenzymatic stoichiometry and nutrient dynamics along a revegetation chronosequence in the soils of abandoned land and Robinia pseudoacacia plantation on the Loess Plateau, China [J]. Soil Biology and Biochemistry, 2019, 134: 1−14. DOI: 10.1016/j.soilbio.2019.03.017
[51] 曾泉鑫, 张秋芳, 林开淼, 等. 酶化学计量揭示5年氮添加加剧毛竹林土壤微生物碳磷限制 [J]. 应用生态学报, 2021, 32(2):521−528. ZENG Q X, ZHANG Q F, LIN K M, et al. Enzyme stoichiometry revealed that five years nitrogen addition aggravated the carbon and phosphorus limitation of soil microorganisms in a Phyllostachys pubescens forest [J]. Chinese Journal of Applied Ecology, 2021, 32(2): 521−528.(in Chinese)
[52] 钟泽坤, 杨改河, 任成杰, 等. 黄土丘陵区撂荒农田土壤酶活性及酶化学计量变化特征 [J]. 环境科学, 2021, 42(1):411−421. ZHONG Z K, YANG G H, REN C J, et al. Effects of farmland abandonment on soil enzymatic activity and enzymatic stoichiometry in the Loess Hilly region, China [J]. Environmental Science, 2021, 42(1): 411−421.(in Chinese)
-
期刊类型引用(2)
1. 李佳思,刘迎庆,张永恒,张迎澳,肖烨子,刘露,余有本. 茶树CsNCED2启动子互作转录因子筛选及在非生物胁迫中的响应. 茶叶科学. 2023(03): 325-334 .  百度学术
百度学术
2. 赵彩良,张洁,唐锐敏,贾小云. 甘薯块根cDNA酵母文库的构建及IbNCED3启动子互作蛋白的筛选鉴定. 山西农业大学学报(自然科学版). 2022(04): 19-27 .  百度学术
百度学术
其他类型引用(2)




 下载:
下载: